Abstract
The convergence of nanomedicine and genetics is reshaping modern healthcare. This article explores how mRNA technologies, genetic tools, and nanoparticle delivery systems are driving breakthroughs in cancer, infectious diseases, and rare disorders, paving the way for faster, more precise therapies.
First-in class science leads to the convergence of nanomedicine and genetics
In a comment published in the current March issue of Nature Nanotechnology, J. Conde, R. Langer and J. Rueff went back on the historical convergence which is currently leading to a health revolution [1].
By combining advanced genetic tools such as non-immunogenic modified mRNA (Karikó, Weissmann, 2005), CRISPR-Cas9 mRNA editing and the tremendous scientific effort performed in nanocarriers, new therapies in oncology, and genetic, cardiovascular or infectious diseases became closer than ever.
The authors duly acknowledge almost a century of scientific breakthroughs from first-in-class researchers and laboratories enrooted in the fundamental discovery of the double helix DNA structure by Franklin, Watson & Crick in 1953 and the RNA identification as an intermediary molecule by Sydney Brenner in 1961…both awarded by the Nobel Academy.
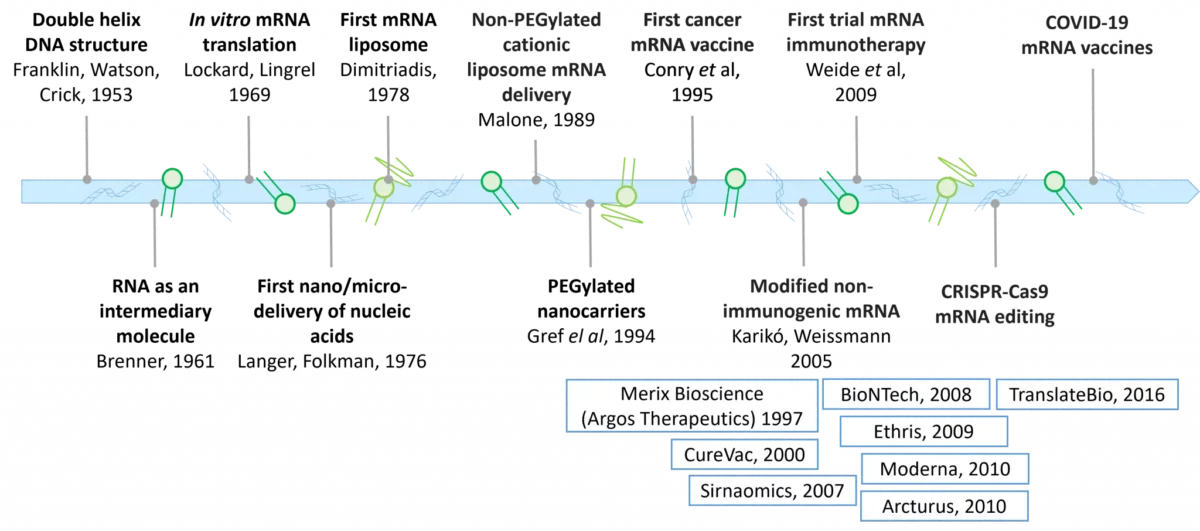
Since Merix Bioscience, now Argos Therapeutics, the first mRNA company has been founded in 1997, a series of now worldwide known biotech and pharmtech have contributed to transfer some of these scientific breakthrough into nanomedicines. The growing and very dynamic ecosystem of the field, from academic laboratories to biotech and major companies illustrates how important is the convergence of genetics and nanomedicines.
What’s next?
According to the ClinicalTrials.gov dataset, more than 200 mRNA-based vaccines are tested in clinical trials for non COVID diseases. Authors claim that the in vitro relatively easiness of mRNA manufacturing will allow already operating pharmaceutical industries manufacturers to massively produce mRNA vaccines. Still, manufacturing the final product (liposome or LNP containing mRNA) through efficient and capable to scale techniques remains a challenge. For instance, solutions to improve lipid nanoparticle stability and multifunctionality have to be found. Recent microfluidic liposome and mRNA lipid nanoparticle fabrication methods address these needs by bringing a fine control over the physical properties of the nanoparticles (size, homogeneity as well as reproducibility).

Are you interested in microfluidics methods to manufacture nanomedicines?
Reach out to us to learn how we can help!
References
[1] Conde, J., Langer, R. & Rueff, J. mRNA therapy at the convergence of genetics and nanomedicine. Nat. Nanotechnol. (2023). https://doi.org/10.1038/s41565-023-01347-w