An introduction to novel LNP targeting strategies for optimizing RNA-LNP biodistribution and improving cell specificity
LNP targeting has become the key limiting factor for expanding RNA therapeutics beyond liver-centric applications. While lipid nanoparticles enable safe and efficient RNA delivery, their default hepatic biodistribution restricts broader clinical use. This review examines the biological drivers of liver tropism and highlights four complementary LNP targeting strategies—selective organ targeting, active ligand-based targeting, next-generation ionizable lipid design, and hybrid LNP platforms—positioning LNP targeting as a central design parameter for next-generation RNA medicines.
RNA therapeutics: From concept to clinical reality
Over the past decade, RNA therapeutics have moved from theoretical promise to clinical reality, with nearly a dozen RNA-based drugs and mRNA vaccines now approved by the FDA and hundreds currently in clinical trial.
The potential is huge as messenger RNA (mRNA), small interfering RNA (siRNA), gene-editing platforms combining Cas9 and guide RNA, and emerging RNA modalities offer a programmable and modular therapeutic framework. These technologies enable transient protein expression, gene silencing, gene editing, or immune and cellular reprogramming without unwanted genomic modification, a key safety and flexibility advantage over DNA-based approaches and a backbone of the next generation of gene therapies.
However, RNA molecules cannot function alone in vivo. They are intrinsically unstable, rapidly degraded by nucleases, and unable to cross cellular membranes. As a result, the clinical success of RNA medicines is inseparable from the performance of their delivery systems.
Lipid Nanoparticles as the backbone of RNA therapeutics
In this context, lipid nanoparticles (LNPs) have rapidly emerged as the leading mRNA delivery, and more generally RNA delivery, platform for in vivo RNA therapeutics thanks to their greater safety and efficacy than viral vectors alternatives, the current standard for gene therapies. Their clinical success—illustrated by the first approved mRNA vaccines and multiple RNA drugs targeting hepatic indications—has firmly established LNPs as a clinically validated solution for RNA delivery.
Beyond their biological performance, RNA–LNP platforms offer strong advantages in terms of safety, manufacturability, and process robustness. The extensive clinical and real-world use of LNPs, most notably through the administration of more than nine billion doses of mRNA COVID-19 vaccines worldwide, has demonstrated a favorable safety profile at unprecedented scale. [1]
From a manufacturing perspective, LNP formulations routinely achieve RNA encapsulation efficiencies exceeding 90%, resulting in a high proportion of functional particles. This intrinsic efficiency translates into superior batch-to-batch consistency, improved dose efficiency, and greater robustness during scale-up. As a result, RNA–LNP technologies are particularly well suited for industrialization, late-stage development, and global deployment.
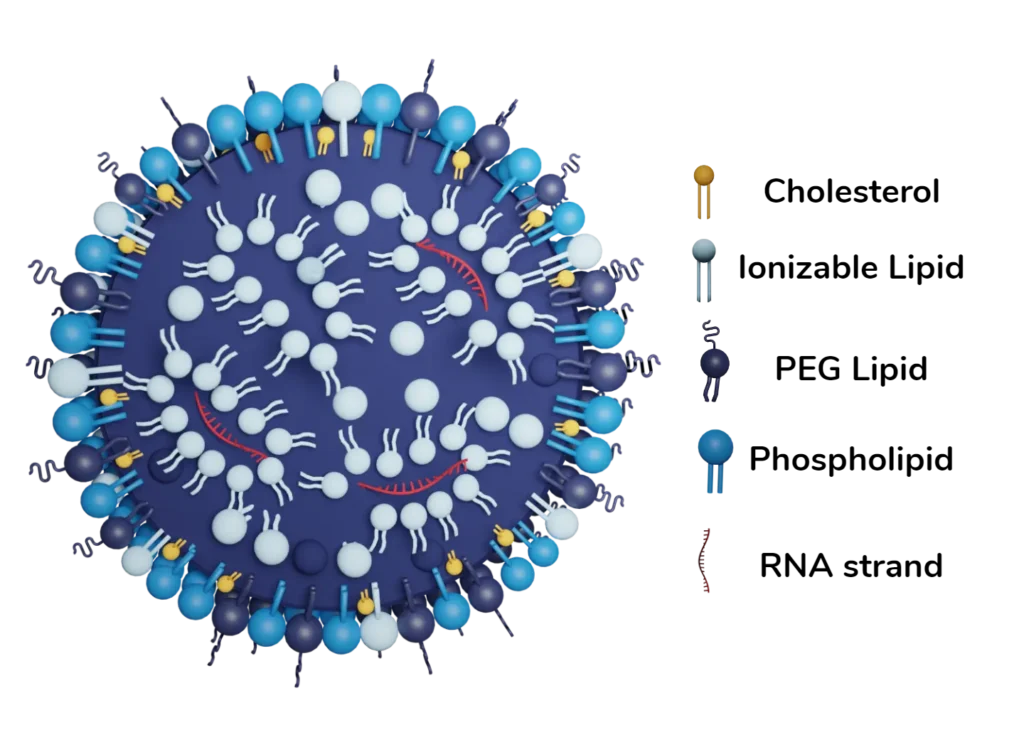
LNP biodistribution & cell specificity as the remaining limiting factor
Nevertheless, while the tremendous potential of RNA–LNP therapeutics is undeniably huge, their future clinical expansion hinges on a single critical challenge: the ability to reliably deliver RNA beyond the liver.
Following systemic administration, most current LNP formulations exhibit a strong hepatic tropism. While this property has enabled remarkable success in liver-targeted indications, it also restricts access to many high-value therapeutic applications, including immune cell engineering, muscle diseases, cardiovascular disorders, and central nervous system pathologies.
Consequently, the fine control of LNP biodistribution through LNP targeting has emerged as the central technological challenges in the field. How precisely RNA–LNPs can be directed to specific tissues and target cell types, such as T cells or stem cells, will ultimately determine how far this platform can extend beyond its current clinical scope.
Expanding promises: from liver diseases to in vivo cell engineering
Due to the incredible potential of RNA technology, LNPs are increasingly explored for numerous applications outside vaccines and liver-targeted indications such as:
- in vivo CAR-T cell generation
- transient immune cell reprogramming
- treatment of genetic muscle disorders
- cardiovascular and inflammatory diseases
- oncology and precision immunotherapy
In principle, RNA-LNP are ideally suited for these indications as they enable transient expression, avoid permanent genome modification, and can be redosed. Moreover, the same LNP platform could potentially be reused across programs by simply changing the RNA cargo.
However, despite these theoretical advantages, clinical translation remains uneven. The key reason behind: LNP biodistribution.
The core bottleneck: default hepatic biodistribution
Under systemic administration, current LNPs display a strong hepatic tropism. In practice, this means that the majority of injected particles accumulate in the liver, regardless of the intended target tissue.
This default LNP biodistribution results from a combination of factors:
- particle size and surface charge
- the adsorption of apolipoprotein E (ApoE) onto the LNP surface (protein corona) enabling recognition by low-density lipoprotein receptors (LDLRs) highly expressed on hepatocytes, thereby promoting selective liver accumulation
- clearance mechanisms of the mononuclear phagocyte system (MPS)
Consequently, LNPs are highly efficient at transfecting hepatocytes, but much less effective in other tissues. Delivery to T cells, muscle, heart, central nervous system, or circulating immune cells hence remains naturally limited.
As a direct consequence, LNP targeting has become the primary limiting factor for expanding RNA therapeutics beyond liver-centric indications. Escaping this “liver-by-default” behavior is now the defining challenge of the field.
LNP targeting strategies: Four complementary approaches to control biodistribution
Multiple LNP targeting strategies are currently under active development to improve targeted delivery and RNA delivery efficiency in vivo. Each approach presents specific trade-offs in terms of CMC complexity, LNP formulation robustness, scalability, and clinical translation. To date, no single strategy has emerged as a universal solution, and different therapeutic objectives may require different targeting paradigms.
Together, these approaches illustrate how LNP targeting has become a central design parameter in RNA therapeutics and mRNA delivery.
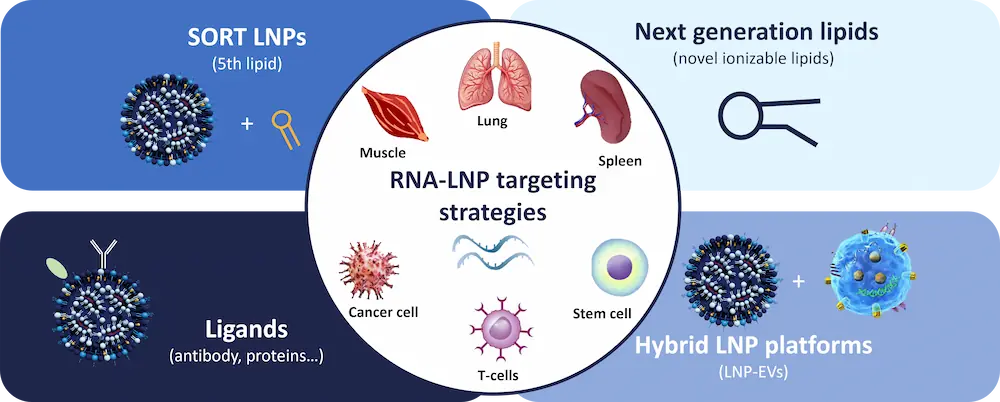
1. SORT LNPs: engineering organ-level biodistribution
The SORT LNP (Selective Organ Targeting LNP) strategy represents one of the first rational and formulation-driven approaches to reprogram LNP biodistribution.
SORT LNPs rely on the controlled incorporation of auxiliary ionizable or charged lipids—often referred to as a “fifth lipid”—into the LNP formulation. By modulating the LNP composition, SORT LNPs can bias LNP delivery toward specific organs such as the lung, spleen, or vascular endothelium. Importantly, this form of passive targeting is achieved without surface modification or external targeting ligands.
While SORT LNPs do not yet enable true cell-specific targeting at the level of individual target cells, they demonstrate a critical principle: LNP targeting and biodistribution are not fixed properties but can be engineered through formulation design. Current SORT LNP data remain largely preclinical, but the approach is attractive due to its strong compatibility with scalable manufacturing.
From a process standpoint, RNA–LNP formulation for SORT LNPs is straightforward, as it follows the same microfluidic or mixing processes used for traditional four-component LNPs. This makes SORT LNPs particularly appealing from a CMC and scale-up perspective.
2. Active LNP targeting via surface ligands
In contrast to passive approaches, active targeting strategies aim to achieve cell-level specificity by functionalizing the LNP surface with targeting ligands that bind defined cellular receptors.
These ligands may include antibodies, scFv fragments, aptamers, peptides, or sugars, enabling targeted LNP delivery to specific cell populations. Recent studies have demonstrated successful T cell targeting, myeloid cell targeting, and immune-cell-specific mRNA delivery in vivo, highlighting the potential of active targeting for advanced RNA therapeutics.
However, active LNP targeting significantly increases formulation complexity. Surface modification alters particle stability, protein corona formation, pharmacokinetics, immune response, and cellular uptake. As a result, active targeting requires careful balancing between delivery efficiency, therapeutic efficacy, and manufacturability.
Depending on the ligand type, LNP formulation strategies may vary, including:
- co-formulation of the ligand with the aqueous phase,
- pre-functionalization of a lipid component (commonly the PEG-lipid),
- or post-formulation surface conjugation.
Despite these challenges, ligand-based active targeting remains one of the most powerful strategies to overcome non-hepatic LNP delivery barriers, particularly for immune cells and circulating target cells.
3. Next-generation ionizable lipid chemistry
A third, increasingly dominant LNP targeting strategy focuses on the design of next-generation ionizable lipids.
The objective is to move beyond widely used lipids such as MC3 LNP, ALC-0315, or SM-102, and instead exploit endogenous targeting mechanisms driven by lipid chemistry. This approach leverages passive targeting, where subtle variations in lipid pKa, hydrophobicity, and chain architecture influence LNP uptake, cellular uptake pathways, endosomal escape, and delivery efficiency.
Through rational lipid design, next-generation RNA–LNP formulations have demonstrated improved biodistribution and functional mRNA expression in tissues such as muscle, spleen, and endothelial cells. These effects are mediated in part by altered interactions with serum proteins, protein corona composition, and Kupffer cell uptake.
Critically, this strategy avoids biological ligands and remains fully compatible with GMP manufacturing, making it one of the most realistic short-term paths toward second-generation LNP therapeutics.
As with traditional LNP, the RNA-LNP formulation process remains unchanged, relying on standard nanoparticle formulation and mixing technologies.
4. Hybrid LNP platforms combining biological components
Finally, hybrid LNP targeting platforms aim to combine lipid nanoparticles with biological elements such as exosomes, virus-like particles, or microenvironment-responsive coatings.
These systems seek to exploit natural cellular uptake pathways while retaining the flexibility of RNA delivery and mRNA expression. Hybrid platforms may enhance delivery to difficult-to-transfect cells or improve targeting in complex biological environments, including tumors.
However, hybrid LNP formulations are substantially more complex than conventional LNP technologies. They often involve multi-step manufacturing processes, increased variability, and significant scale-up challenges. As a result, most hybrid LNP platforms remain early-stage and preclinical, with open questions regarding CMC, regulatory acceptance, and large-scale production.
Conclusion – LNP targeting will define the next generation of RNA therapeutics
Hepatic LNP targeting is now well controlled and clinically validated. However, expanding RNA therapeutics beyond the liver will depend on our ability to precisely engineer LNP targeting and biodistribution. While myeloid and T cell targeting are advancing rapidly, delivery to the central nervous system and solid tumors remains particularly challenging.
Crucially, LNP targeting alone is not sufficient. Therapeutic efficacy ultimately depends on intracellular trafficking, translation efficiency, and cell-state biology. In this context, RNA design—through regulatory elements such as microRNA-responsive sequences—offers a complementary lever to refine functional biodistribution. In the coming years, progress in LNP targeting and RNA engineering will be the decisive factor that transforms RNA therapeutics from liver-focused solutions into truly systemic, multi-organ medicines.

Looking to formulate targeted RNA-LNP?
Reach out to us to learn how we can help!
References
[1] Semenzato L, Le Vu S, Botton J, et al. COVID-19 mRNA Vaccination and 4-Year All-Cause Mortality Among Adults Aged 18 to 59 Years in France. JAMA Netw Open. 2025;8(12):e2546822. doi:10.1001/jamanetworkopen.2025.46822. Available at https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2842305