Home Resources Application notes
Liposomes synthesis and size characterization with high precision DLS
This joint work by Cordouan Technology and Inside Therapeutics presents cutting-edge techniques for formulating and characterizing lipid-based nanoparticles.
Abstract
The application note introduces how the TAMARA microfluidics platform allows for precise control in liposome production, ensuring optimal size and Polydispersity Index (PDI) control, crucial for drug delivery. Characterization of size and PDI is achieved through the utilization of the VASCO Kin DLS system, which provides real-time monitoring and consistent quality assurance for liposome samples, a key factor for more efficient nanoparticle-based delivery.
TOMATE
Introduction
Liposomes, spherical vesicles composed of lipid bilayers, have emerged as versatile and indispensable tools in various fields of science and medicine (Figure 1). Their unique structure allows for the encapsulation of hydrophilic and lipophilic compounds, making them ideal candidates for drug delivery systems, cosmetics, and diagnostic applications [1,2]. The ability to tailor liposomes to encapsulate specific payloads, control release kinetics, and enhance bioavailability has them a key component in pharmaceutical research and clinical practice.
Despite their numerous advantages, the traditional methods of liposome synthesis present several challenges. Conventional techniques often involve batch processes with limited control over size distribution, leading to batch-to-batch variability. Homogenization methods may result in low encapsulation efficiency and compromised structural integrity. These challenges underscore the need for innovative technologies to overcome the limitations of traditional liposome manufacturing methods [3].
In recent years, microfluidic platforms have gained prominence as a promising alternative for liposome synthesis. The precise control over fluid dynamics in microchannels allows for uniform mixing of lipids and encapsulated compounds, resulting in highly monodispersed liposomes population with controlled size and properties. The microfluidic approach addresses issues related to scalability, reproducibility, and particle size distribution, offering a more robust and efficient solution for liposome production.

In this context, we present TAMARA – a groundbreaking microfluidic mixing platform designed to revolutionize liposome synthesis (Figure 2). By leveraging the capabilities of TAMARA, researchers and manufacturers can achieve unparalleled control over liposome characteristics, paving the way for advancements in drug delivery, diagnostics, and cosmetic formulations.
In the subsequent sections of this application note, we will delve into the operational principles of TAMARA and showcase its efficacy in overcoming the challenges associated with traditional liposome manufacturing methods. Through detailed experiments and analyses, we aim to demonstrate the superior performance and versatility of TAMARA in the synthesis of liposomes, providing a glimpse into the future of precision-engineered liposome technologies.

How does liposome synthesis work?
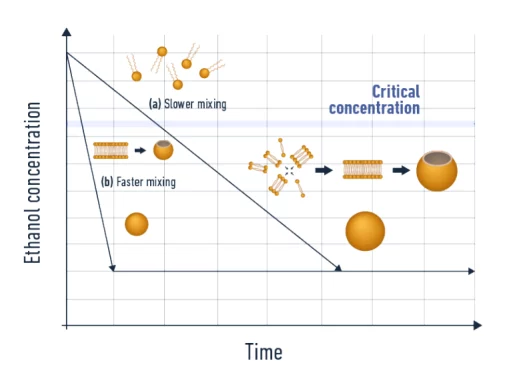
The fundamental principle of liposome synthesis by self-assembly relies on the fast mixing of two miscible phases (lipids in an organic solvent and aqueous buffer), leading to a drop in solubility of the lipids, which start to agglomerate to form nanoparticles (Figure 3).
As one can observe, the mixing speed has a major influence on the synthesized nanoparticle size [5]. The faster the mixing, the smaller the nanoparticle is. Also, the more efficient and homogeneous the mixing is, the better the control over the size and distribution.
Therefore, ensuring a uniform and constant mixing time throughout the process is crucial to optimize the final nanoparticle size control and homogeneity. More information can be found in our organic nanoparticle formation review.
In this context, microfluidics is the ideal solution thanks to its unique ability to maintain laminar flow condition conditions, thus permitting excellent reproducibility of the mixing condition for optimal control of the final nanoparticle physicochemical parameters.
Physico-chemical characterization of liposomes
The size of nanoparticles stands out as a critical quality attribute (CQA) that exerts influence on stability, encapsulation efficiency, biodistribution, and cellular uptake. The endocytosis process of nanoparticles into cells varies greatly depending on their size, and the optimal size is contingent on factors such as the payload, target cell, and experimental model (whether in vitro, mice, primate, etc.). Typically, lipid nanoparticles (LNPs) fall within the range of 60 to 120 nm, avoiding issues such as liver clearance and kidney filtration associated with overly large particles (>200 nm), and toxicity concerns linked to excessively small particles. TAMARA introduces a unique capability to finely control nanoparticle size, enabling the precise optimization of drug release efficiency.
In the realm of nanoparticle characterization, size can be defined in two ways: hydrodynamic diameter (often measured via Dynamic Light Scattering, DLS) or core diameter (typically assessed through Cryo-Transmission Electron Microscopy, Cryo-TEM). The Polydispersity Index (PDI) emerges as a crucial parameter describing the degree of non-uniformity within a nanoparticle population. Its significance extends to both drug delivery efficiency and toxicity. Represented as (Standard deviation/mean size)2, a lower PDI value indicates a more monodisperse population. While FDA guidelines suggest maintaining a PDI below 0.3 for liposomal drug products [4], smaller values are preferred. Leveraging advanced microfluidics technology, TAMARA achieves exceptional PDI levels within a formulation, typically around 0.1 for siRNA-LNP.

Particles in suspension undergoing Brownian motion interact with the laser beam and from the scattered light we can extract the diffusion coefficient and hydrodynamic diameter of the particles.
Widely used technique for the size characterization of liposomes. It operates on the principle of measuring fluctuations in the scattering of laser light caused by particles undergoing Brownian motion in a suspension. The rate of these fluctuations is related to the size of the particles, allowing for the determination of the hydrodynamic radius of liposomes (Figure 4). One key advantage of DLS is its ability to characterize nanoparticles in a wide range of sizes quickly and non-invasively, making it an effective tool for analyzing liposomal suspensions.
Several studies have highlighted the utility and effectiveness of DLS in liposome characterization. For instance, Surianarayanan et al. (2016) emphasized the significance of liposome concentration during DLS measurement for obtaining reliable results. This study underlines the importance of understanding how factors like liposomal dilution can impact the characterization outcomes in DLS [6].
Liposomes synthesis with the TAMARA platform
The lipid mixture used in the following experiments was composed of phosphatadylcholine (Lipoid S100, Lipoid Gmbh) and Cholesterol (Sigma-Aldrich) at a mass ratio of 2:1 respectively. A stock solution of 60 mg/mL Lipoid:Chol was prepared by carefully weighing each lipid powder, resuspending it in absolute ethanol and using sonication to dissolve all lipids. The working solution was prepared at 10 mg/mL, by diluting the stock solution. Demineralized water was employed as the aqueous phase. The synthesis was carried out at room temperature with the TAMARA platform.
Briefly, after choosing the synthesis conditions on the controller unit, each reagents was pipetted into the reusable réservoirs. After a few seconds of synthesis, the resulting liposome sample was collected and kept at 4°C until the measurement. All samples were diluted 20 times in DI water prior measurement by DLS with the VASCO Kin instrument..

The VASCO Kin features a sensitive single photon Avalanche Photodiode Detector (APD) and fast acquisition electronics for real-time monitoring of light intensity fluctuations. Its patented software correlator enables precise nanometric size measurements, as low as 1 nm, under both static and dynamic conditions, while minimizing interference from intermittent events like
dust.
In addition, the VASCO Kin has been designed to adapt to a wide range of applications thanks to its different measuring heads connected by optical fibers. In particular, the “in-situ head” was used for these experiments (Figure 5). This device is a remote measurement probe specifically designed to enable direct and contactless measurements, eliminating the requirement for sample batching processes. It offers versatile and innovative ways to use DLS, like in-situ monitoring of particles synthesis directly in reactors, or millifluidic in line measurement.
Batch-to-batch repeatability analysis
In this experiment, the synthesis conditions were kept constant with a flow rate ratio of 3 : 1 and a total flow rate of 4 mL/min. This was repeated at different final target volume (0.25, 1 and 10 mL) and in triplicates. The same chip was used during this study and careful washing steps were performed between each run.


channel in the TAMARA platform. (A) Size and PDI obtained in 3 consecutive runs at different target
volumes. (B) Correlograms and (C) size distributions highlight the monodispersity and repeatability
at 1 mL target volume.
We have found an excellent repeatability between all replicates, regardless of the target volume (Figure 6). The relative standard deviation of the size at 0.25, 1 and 10 mL is equal to 6.5, 5.5 and 2.5 % respectively. This correspond to a max size deviation of about 4 nm. Knowing that the maximum repeatability of the DLS measurement is 5%, these differences in size between batches are low to negligible.
All samples were homogeneous with a PDI below 0.3 at 250 µL and below 0.2 in all other cases. Higher PDI at smaller volumes can be explained by the increased proportion of « head » and « tail » of the sample, where mixing conditions are not optimal.
Real-time Dynamic Light Scattering monitoring
Utilizing the software accompanying the VASCO Kin instrument (NanoKin) enables real-time monitoring of the sample in situ. In the provided example (see Figure 7), an unexpected event occurred during a 1-minute measurement. At the time of this event, we were able to visually identify the source of this anomaly.
Typically, dust and fibers serve as common contaminants in a non-clean room environment. These particles, typically in the µm size range, can significantly distort DLS results. Fortunately, the software offers the capability to selectively eliminate these artifacts, enabling measurements solely from the sample. Moreover, automatic detection of these interferences is possible, ensuring smooth measurements without concerns of dust or fibers compromising accuracy.

visible on the intensity recording.
Conclusion
In conclusion, the TAMARA microfluidic platform significantly enhances the synthesis of liposomes, offering a high degree of control over their size and properties. This advancement addresses the limitations found in traditional methods of liposome production. One of the key benefits of TAMARA is its capacity to consistently produce liposomes with an optimal Polydispersity Index (PDI), crucial for the efficacy and safety of drug delivery systems. Furthermore, the VASCO Kin real-time monitoring and batch-to-batch repeatability analysis ensure the production of uniform
and high-quality liposome samples, essential for pharmaceutical and medical applications. This establishes TAMARA and VASCO Kin as superior technologies in the field of liposome synthesis and characterization, paving the way for more effective and reliable drug delivery solutions.

References
[1] Chaves, M. A., Ferreira, L. S., Baldino, L., Pinho, S. C., & Reverchon, E. (2023). Current Applications of Liposomes for the Delivery of Vitamins: A Systematic Review. In Nanomaterials (Vol. 13, Issue 9). MDPI.
https://doi.org/10.3390/nano13091557
[2] Ahmadi Ashtiani, H. R., Bishe, P., Lashgari, N.-A., Nilforoushzadeh, M. A., & Zare, S. (2016). Liposomes in Cosmetics. Journal of Skin and Stem Cell, 3(3). https://doi.org/10.5812/jssc.65815
[3] Liu, P., Chen, G., & Zhang, J. (2022). A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. In Molecules (Vol. 27, Issue 4). https://doi.org/10.3390/molecules27041372
[4] U.S. Department of Health and Human Services Food and Drug Administration, & (CDER), C. for D. E. and R. (2018). Liposomes Guidance for Industry. In Guidance for Industry (Issue April).
http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.html
[5] Maeki, M., Fujishima, Y., Sato, Y., Yasui, T., Kaji, N., Ishida, A., Tani, H., Baba, Y., Harashima, H., & Tokeshi, M. (2017). Understanding the formation mechanism of lipid nanoparticles in microfluidic devices with chaotic micromixers. PLOS ONE, 12(11), e0187962. https://doi.org/10.1371/journal.pone.0187962
[6] Surianarayanan, R., Gurumallappa Shivakumar, H., Varma Vegesna, N. S. K., & Srivastava, A. (2016). Effect of sample Concentration on the Characterization of Liposomes using Dynamic light Scattering Technique. Pharmaceutical Methods, 7(1), 70–74. https://doi.org/10.5530/phm.2016.7.11
About us…
Inside Therapeutics (Inside Tx) is a french start up based in Bordeaux (France). The company designs, develops and markets instruments and services for manufacturing RNA-based lipid nanoparticles and liposomal products.
These nanomedicines are used to combat genetic diseases, cancers and infectious diseases (RNA vaccines are lipid nanoparticles). The first instrument developed by the company enables academic or private laboratories (biotechs/pharmas) to manufacture and test candidate nanomedicines. The company’s ambition is to become a major player in the nanomedicine biomanufacturing sector in the Nouvelle Aquitaine region.
Inside Therapeutics has been founded in 2022 by an experienced team, with a complementary background in biotechnology, nanoparticles, engineering, marketing, business and entrepreneurship. All products are designed and manufactured in France.
Interested ?
Contact us at contact@insidetx.com

Cordouan technologies is a French SME located in Bordeaux (France). The company is specialized in the design, manufacture and commercialization of innovative & advanced solutions for the characterization of nanoparticle suspensions and complex colloidal samples.
Offering advanced and innovative solutions to meet end-user’s needs, including customization of instruments to suit the application, Cordouan will be proud to help you make great developments and
advance science.
After more than 15 years of continuous progress and innovation, these instruments are now used by many renowned academic research laboratories and famous industrial companies in a very wide range of applications, such as microbiology, life sciences, bio-pharma, cosmetics, nano-medicine, petroleum, energy
production, transformation and storage, functionalized inks & printing, nanopolymers, environment, etc.
All products are designed and manufactured in France.
Interested ?
Contact us at contact@cordouantech.com

Save the application note
Full PDF
Looking to get started or improve your LNP formulation screening?
Reach out to us to discover how we can help!
Other Application notes
Looking for more detailed application notes on nanoparticle formulations? Check out our other resources!
See all Application notes

