Home Resources Application notes
Streamlining mRNA-LNP screening with TAMARA
Evaluating low volumes formulation performance and benchmarking against mainstream toroidal microfluidic mixers.
Abstract
Screening RNA-loaded lipid nanoparticles (RNA-LNPs) is vital for developing effective mRNA vaccines and therapeutics. Microfluidic systems, like TAMARA, are revolutionizing this process by minimizing reagent use and providing precise control over nanoparticle characteristics. Our latest study compares TAMARA with toroidal mixers, revealing superior RNA yield, minimal reagent loss, and up to 200% higher protein expression. TAMARA not only optimizes low-volume formulations but also offers scalability and cost-effectiveness, making it a game-changer in RNA-LNP development.
This study provides a comprehensive evaluation of the TAMARA nanoparticle formulation system, focusing on its performance at low volumes to optimize RNA-LNP formulations while minimizing the use of costly mRNA. Additionally, a comparative analysis is conducted with the widely-used toroidal mixer, allowing for a clear assessment of both systems’ capabilities.
Both systems produced high-quality RNA-LNPs with ideal size and PDI values. The TAMARA system demonstrates strong potential alternative to the toroidal mixer for RNA-LNP formulation, particularly
excelling in encapsulation yield, with virtually no reagent losses, and higher protein expression levels. TAMARA has the ability to reduce reagent consumption while maintaining efficacy and allowing for
chips reusability further underscoring its cost-effectiveness, therefore making it an attractive option for advancing RNA-LNP therapeutic development.
Introduction
The formulation of RNA-loaded lipid nanoparticles (RNA-LNPs) has become a cornerstone in the development of mRNA-based vaccines and therapeutics, thanks to their unique ability to encapsulate RNA and deliver it within the cells. However, developing these mRNA-LNP therapies poses significant formulation challenges, including the need for low-volume screening of lipid compositions and formulation conditions to identify candidates suitable for further in-vivo testing and clinical trials.
Microfluidics-based systems have emerged as a leading approach for RNA-LNP formulation, offering low-volume
formulations that significantly reduce reagent consumption—crucial for cost-effective RNA therapy development—while providing precise control and improved reproducibility over critical nanoparticle characteristics, such as size, PDI, and encapsulation efficiency.
This application note focuses on evaluating the performances of TAMARA, a microfluidic-based nanoparticle formulation system, under various low volume conditions (200 µL, 400 µL, and 700 µL). It aims at determining the extent to which the TAMARA system can minimize reagent consumption while achieving consistent performance by focusing on key RNA-LNP parameters such as particle size, encapsulation efficiency, encapsulation yield, transcription efficiency, cell viability and protein expression levels.
In a second step, TAMARA’s performances have been benchmarked with a common formulation system incorporating a toroidal mixer. This system, previously optimized through a Design of Experiments (DOE) approach, serves as a reference point for comparison.
The findings of this study will provide valuable insights into the potential advantages of the TAMARA system for RNALNP formulation for low volume screening approaches, especially thanks to its virtually 0 losses and low volume control. Please note that this is a preliminary study, and while the data presented herein is promising, further in-depth validation and comprehensive characterization will be necessary to substantiate the claims made.
This work was carried out by the ART “ARNm” team, a R&D Inserm lab led by Prof. Chantal Pichon in Orléans, with significant contributions from Dr. Nabila Laroui and Dr. Milab Baroud, and supported by Inside Therapeutics.
Results
A comprehensive physicochemical characterization and in vitro testing have been conducted to assess the
performance of the two systems. Throughout this study, sample data are labeled according to the following
nomenclature:
For TAMARA:
TAM FRR-TFR Volume (in µL)
Example: For a formulation with an FRR of 4:1,
a TFR of 9 mL/min, and a total formulation
volume of 700 µL, the sample is labeled as:
TAM 4-9 700.
For the toroidal mixer approach:
IGNITE X 700 (where X corresponds to the
sample number previously optimized through a
DOE and 700 corresponding the 700 µL
formulation).
1/ RNA-LNP size & PDI with TAMARA
A/ Impact of formulation parameters
The objective of this initial study is to assess how varying formulation parameters influence the size and
polydispersity index (PDI) of RNA-LNPs produced using
the TAMARA system. Four distinct formulation conditions were used for RNA-LNP formulation at a total volume of 700 µL: TFR 9 mL/min – FRR 4:1, TFR 5 mL/min – FRR 4:1, TFR 9 mL/min – FRR 3:1, and TFR 5 mL/min – FRR 3:1.
Figure 1 shows that all samples formulated with TAMARA exhibit high-quality nanoparticle formulations, with PDI values below 0.2, meeting industry standards.
Additionally, the nanoparticle sizes fall within the optimal range for efficient intracellular delivery (80 to 150 nm).
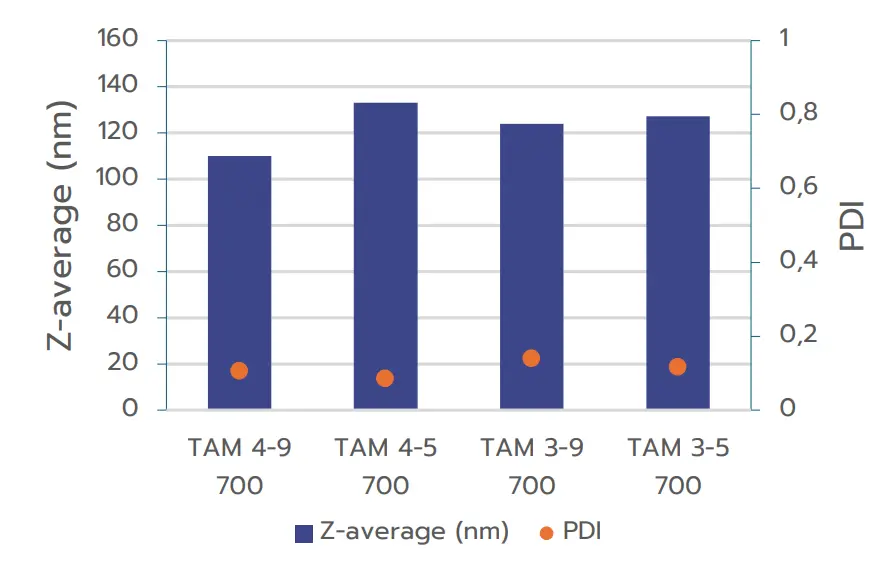
The results illustrate that increasing the Total Flow Rate (TFR) generally leads to a reduction in nanoparticle size, while the Flow Rate Ratio (FRR) has a comparatively smaller impact on nanoparticle size. This effect of the TFR therefore allows for an easy optimization of the final nanoparticle size.
These observations align with the theory of nanoparticle formation through nanoprecipitation, where more efficient mixing between the solvent and aqueous phases—achieved at higher flow rates—results in a greater number of nucleation points and thus the formation of smaller particles. Moreover, rapid mixing reduces lipid agglomeration and particle coalescence. Higher TFRs also generate greater shear forces within the channels, further preventing the formation of larger particles.
B/ Influence of formulation volume
The second study evaluated the impact of formulation volume on nanoparticle size and quality, aiming to identify the minimum formulation volume that TAMARA can process while maintaining high-quality output. Three different total formulations volumes were tested: 200 µL, 400 µL, and 700 µL. Note that as described in introduction, all formulations could not be carried out under the same formulation conditions for each volume. Therefore, we merely compared the flow conditions for the different volumes
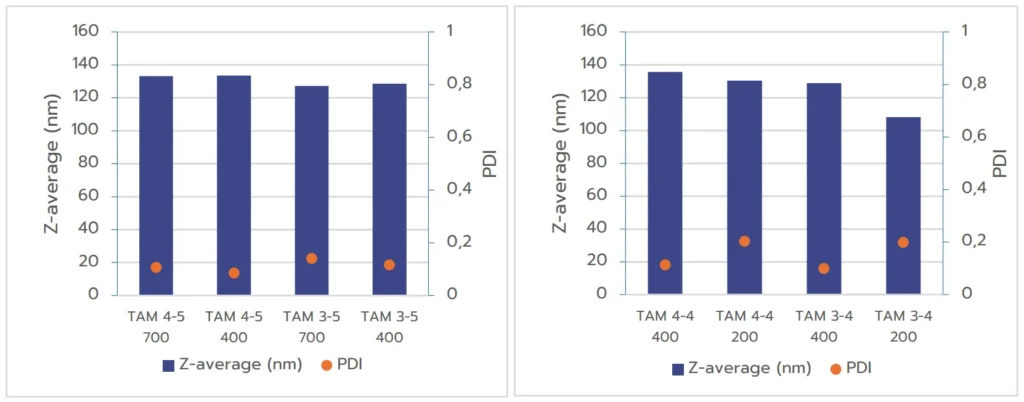
The results shown on figure 2(A) indicate an excellent repeatability in size between the formulation obtained at 700 µL and 400 µL, showing an excellent potential for scalability of the formulation. However, one can note an increase of the PDI at lower volumes.
Figure 2 (B) instead shows that the size of the nanoparticles formed at 400 µL and 200 µL tend to differ quite a lot, indicating that the scalability between those volumes is not necessarily straightforward and requires further
optimization. Moreover, formulations produced at 200 µl display a increased PDI indicating a more inhomogeneous nanoparticle population.
This behavior could be anticipated as smaller batch volumes tend to be more challenging to control, often resulting in broader size distribution therefore a higher PDI. Despite these challenges, the system consistently produced high quality nanoparticles at volumes as low as 400 µL, suggesting that this volume can be an effective starting point for initial screening, with the potential for improved PDI at larger volumes.
C/ Comparison between TAMARA and optimized toroidal mixer
Lastly, considering the previous discussion, we eventually compared nanoparticles formulated using TAMARA at 700 µL and 400µL with those produced using a toroidal mixer under pre-optimized conditions.
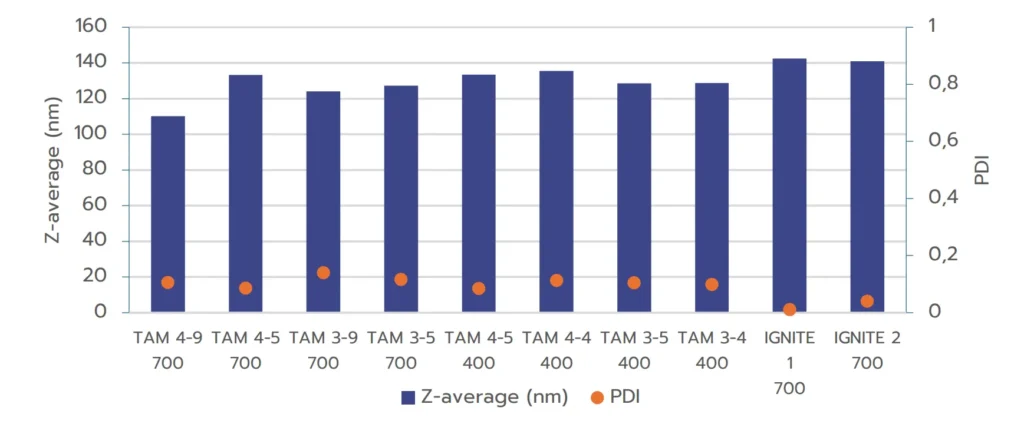
The results in Figure 3 demonstrate that RNA-LNPs formulated with both systems exhibit excellent quality, with size in an ideal size range and PDI below 0.2, meeting regulatory standards. For the TAMARA system, further optimization through a Design of Experiments (DOE) approach may be beneficial for minimizing PDI even further, as shown by the optimized PDI values achieved with the IGNITE formulation.
2/ EE% and EY% study with TAMARA
A/ Impact of formulation parameters
Following the assessment of how formulation parameters influence size-related characteristics of RNA-LNPs, we
assessed their impact on encapsulation efficiency (EE%) and encapsulation yield (EY%). The formulation parameters evaluated here are consistent with those used in the size and PDI studies.
The measurements indicate that the influence of
formulation parameters on encapsulation efficiency is
negligible, with all RNA-LNPs formulated using TAMARA
demonstrating excellent encapsulation efficiencies
exceeding 95%. These results align with the most
commonly reported values in literature.
Additionally, TAMARA shows outstanding encapsulation
yields, reaching up to 95%, surpassing most previously
reported values for RNA-LNPs.1 The sample labeled
TAM 4-9 700 appeared to be an outlier.
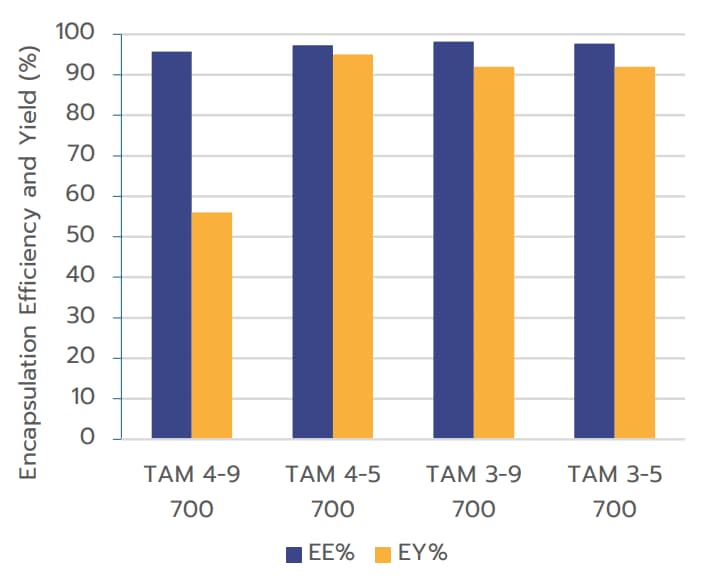
Encapsulation Yield (EY%) when formulated using TAMARA at 700 µL volume
B/ Impact of formulation volume
The effect of the formulation volume on RNA-LNP encapsulation was also evaluated using the TAMARA system. As
previously noted, three total formulation volumes (200 µL, 400 µL, and 700 µL) were tested and nanoparticles
produced under same formulation conditions were compared.
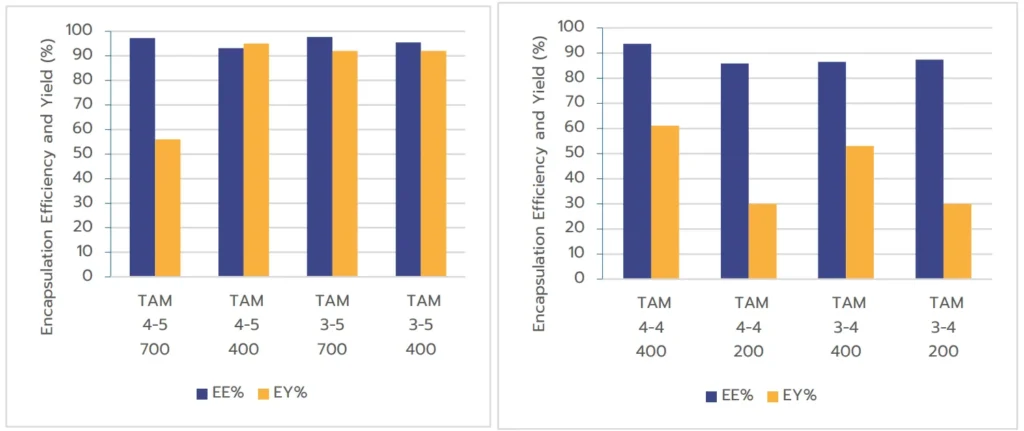
Results in Figure 5 show that EE% remains consistently high across all tested volumes, confirming that TAMARA can effectively maximize RNA-LNP encapsulation efficiency. However, a slight decrease in EE% was observed at the 200 µL volume, dropping from 95% at higher volumes to 85%.
Formulation volume had a more significant impact on EY%. The lowest formulation volume (200 µL) exhibited reduced yield compared to the larger volumes (400 µL and 700 µL).
This reduction is likely due to the extremely small volumes of the two liquid phases (50 µL for lipids, 150 µL for the aqueous phase at an FRR of 1:3), resulting in a very short run time (~3 seconds). In such cases, the timing of mixing becomes critical, and even minor mismatches can significantly decrease the overall yield. In contrast, larger volumes allow for longer formulation times, making the process easier to control and yielding better results. Taking into account this outcome, Inside Therapeutics’ team is currently working on an improved version of TAMARA including an optimized control of formulation parameters at very low formulation volume.
C/ Comparison between TAMARA & Toroidal mixer
To conclude the encapsulation characterization study, we compared the performance of the toroidal mixer with that of the TAMARA system across different volumes and formulation parameters.
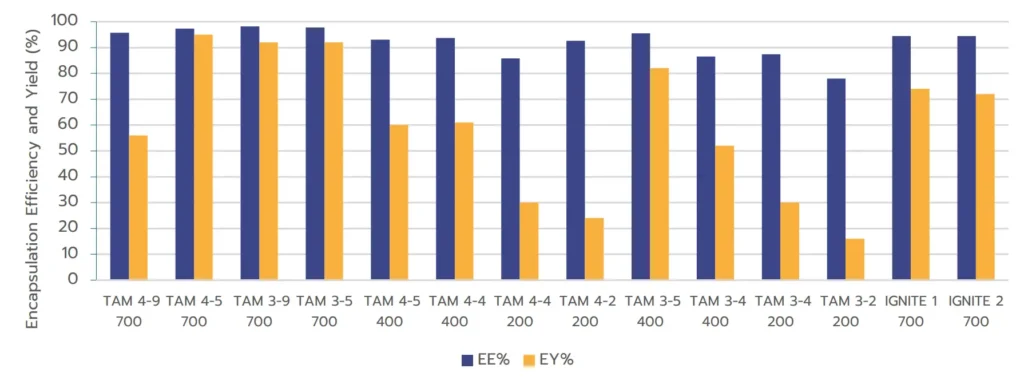
The results indicate that both microfluidic approaches achieve comparable EE%. However, TAMARA demonstrated
significantly superior performance in terms of EY% at 700 µL, consistently achieving yields greater than 90%. In
contrast, the toroidal mixing approach, even under optimized conditions, reached only around 75% yield at a similar volume of 700 µL. Lowering the working volume led to a decrease in yield, as described above.
3/ Impact of formulation parameters on Transcription efficiency, Cell viability & Protein expression
A/ Impact of formulation parameters
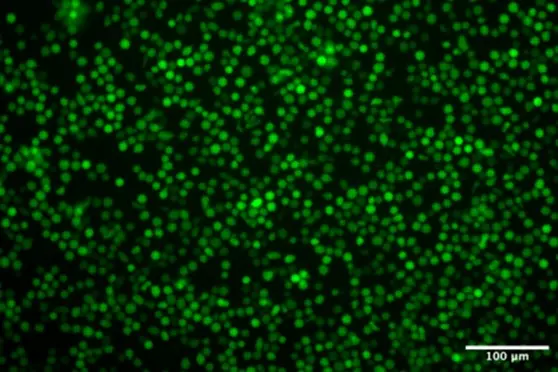
Following the physicochemical characterization of RNA-LNPs produced with TAMARA, an in vitro study was conducted to assess TAMARA’s performance in a biologically relevant context using eGFP encoding mRNA-LNPs.
This phase of the study focused on evaluating the impact of formulation parameters on translation efficiency as a
measure of delivery effectiveness, cell viability (to assess cytotoxicity), and protein expression (using mean
fluorescence intensity of cells as read out).
The results indicate that formulation parameters have
minimal to no effect on transfection efficiency, which
consistently remained near 100%, nor on cell viability,
which remained high with low cell death (<15%).
However, the protein expression levels varied among
samples. The significant difference in mean
fluorescence intensity of eGFP of the first sample (TAM
4-9 700) compared to others is most likely linked to
the lower encapsulation yield previously discussed.
The differences between other samples are likely
linked to the combined effects of encapsulation yield,
nanoparticle size, and other formulation parameters
that may influence cellular uptake.
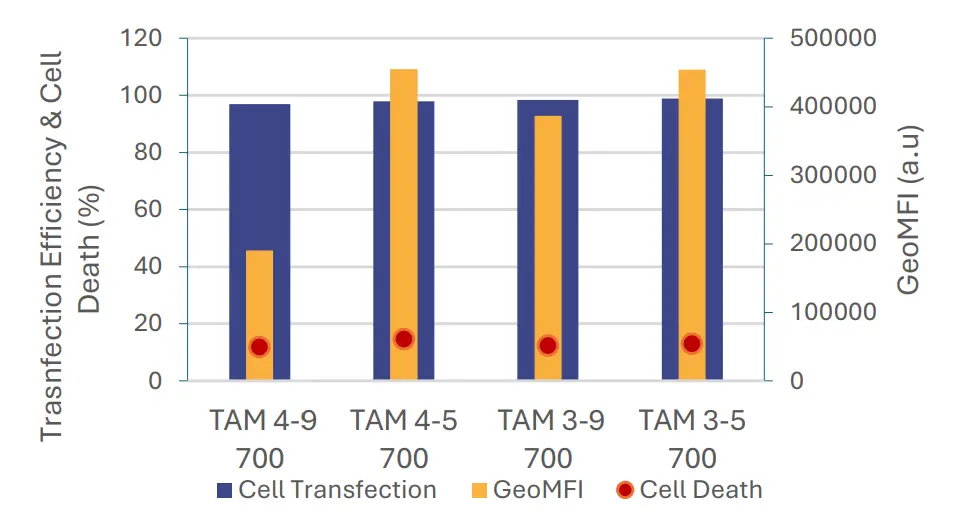
and protein expression following a 16 hours exposure
to mRNA-LNP formulated using TAMARA at 700 µL
volume
B/ Impact of formulation volume
Next, the study examined the impact of formulation volume to determine the minimum viable working volume for an in-vitro study.
Once again we compared data obtained at different volumes under identical flow conditions
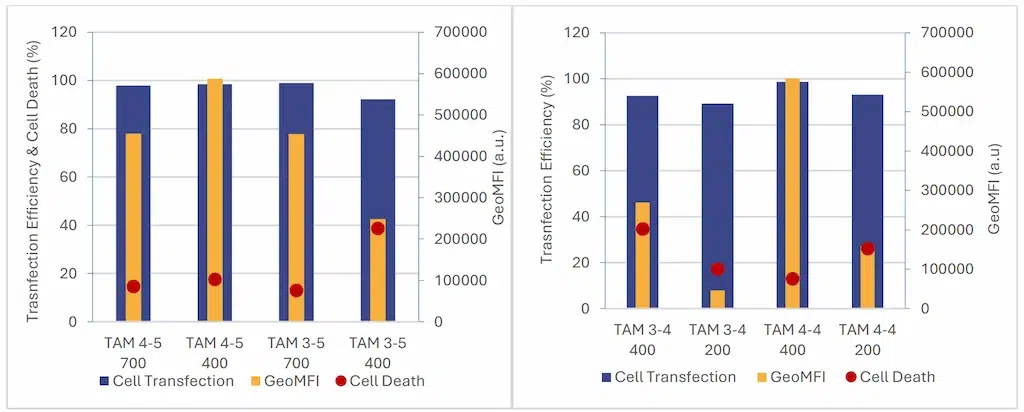
As shown in Figure 8, the transfection efficiency remains high across all samples (>90%). Nevertheless it tends to
slightly decrease with lower volume . Cytotoxicity was minimal at larger volumes but tended to increase with smaller formulations. Those data suggest a strong link between encapsulation yield and cell expression, as formulation parameters leading to higher encapsulation yields also exhibited higher cell expression levels.
These findings align with previous results indicating that lower formulation volumes lead to reduced encapsulation yields, subsequently resulting in decreased overall expression levels in cells.
C/ Comparison between TAMARA & Toroidal mixer
Finally RNA-LNPs formulated using TAMARA were compared to those produced with a toroidal microfluidic mixer
under previously optimized conditions.
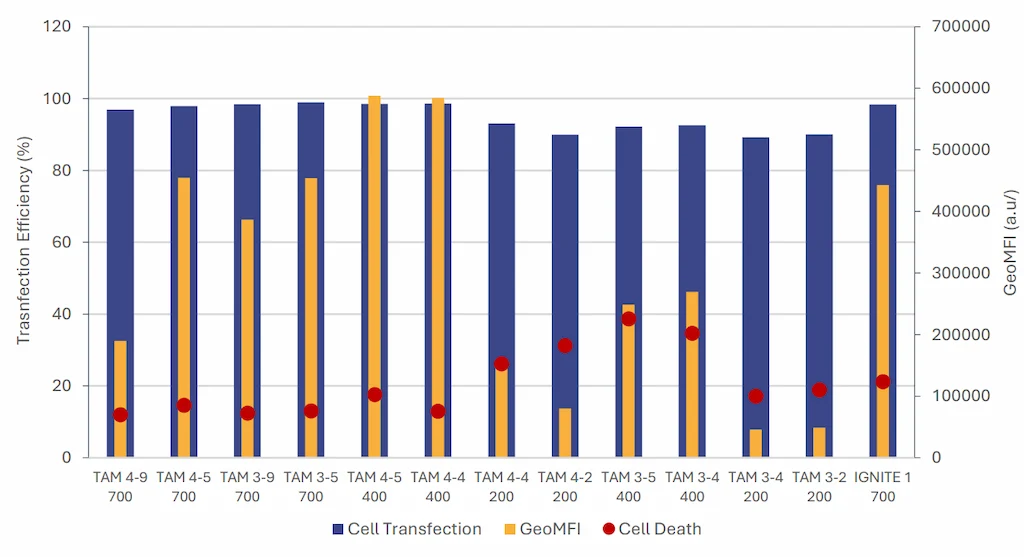

The data show that RNA-LNPs formulated with both TAMARA and the toroidal mixer achieve similar transcription
efficiencies, approaching 100%. Cell viability remained comparable with minimal cytotoxicity observed in both systems.
What is more, RNA-LNPs formulated by TAMARA, even though not optimized, exhibited up to 30% higher expression levels compared to those produced with the IGNITE system, despite the latter being previously optimized for cell expression. This data further highlights the need for thorough optimization studies and efficient formulation systems to maximize protein expression.
It can also be noted that formulation condition (both TFR and FRR) have a major impact on the final in-vitro efficiency further enhancing the need of thorough screening of LNP formulation conditions.
Discussion
This comprehensive study has provided valuable insights into the physicochemical and in vitro characterization of
RNA-LNPs formulated using different microfluidic approaches with both the toroidal mixer under pre-optimized
conditions and the TAMARA system with varying synthesis parameters and volumes.
In terms of physicochemical characterization, both TAMARA and the toroidal mixing system demonstrated strong
performance, meeting regulatory standards (PDI < 0.2) and effectively controlling nanoparticle size, showcasing the advantages of microfluidic technology.
When assessing the impact of formulation volumes, the study highlighted that while the 200 µL volume with TAMARA posed some challenges, particularly in terms of lower encapsulation yields, the 400 µL and 700 µL volumes produced consistent, high-quality results. This suggests that a 400 µL formulation volume is an optimal starting point for screening studies, striking a balance between material efficiency and product quality, before potentially being scaled-up for larger scale/in-vivo studies.
In terms of encapsulation efficiency, the TAMARA system offered similar performances to the toroidal mixer. However, when it comes to encapsulation yield, the TAMARA outperformed toroidal mixer approach. Notably, TAMARA achieved encapsulation yields exceeding 90% at volumes as low as 400 µL, whereas the toroidal mixer, even under optimized conditions, managed only around 75% yield at a higher volume of 700 µL. This further highlights TAMARA’s capability as a highly efficient system for RNA-LNP formulation, especially for screening at reduced volumes as the system is maximizing the reagent usage with virtually 0 losses.
Data obtained from in cell studies further reinforced TAMARA’s legitimacy. Both systems exhibited similar high
transcription efficiencies, near 100%, and low cytotoxicity, indicating minimal cytotoxicity. In addition to this, RNA-LNPs formulated with TAMARA achieved up to 30% higher protein expression level than those produced with the Ignite system by Precision Nanosystems, though previously optimized. This suggests that TAMARA not only matches but may surpass the performance of existing toroidal microfluidic mixers in delivering functional RNA to cells.
Future work could involve further characterization to reduce the minimum screening volume for optimal results, and a comprehensive Design of Experiments (DOE) approach could help identify TAMARA’s optimal operating parameters for this RNA-LNP formulation. Nonetheless, even without extensive pre-optimization, TAMARA outperforms toroidal mixers, while significantly reducing the consumption of expensive RNA material.
Moreover, the entire study with TAMARA was conducted using only two chips, highlighting its cost-effectiveness for screening. Additionally, TAMARA offers scalability to volumes in the tens of milliliters while maintaining the same high performance.
Conclusion
In summary, the TAMARA system stands out as a highly effective, and potentially superior, alternative to the Ignite by Precision Nanosystems and other toroidal micro mixers approaches. Its capability to deliver exceptional results across a broad volume range, coupled with the advantages of lower required volumes and reusable chips, makes it an attractive solution for enhancing the RNA-LNP therapeutics research while also improving its accessibility.
Material and methods
RNA-LNP Formulation
RNA-LNP composition
RNA : 5moU eGFP mRNA cleancap1
Lipid Composition : Proprietary LNP from ART
“ARNm” lab
RNA-LNP formulation systems
Two distinct nanoparticle formulation systems, each
utilizing a different microfluidic mixing technology,
were employed in this study with the objective of
benchmarking these two approaches.
TAMARA
Tamara is an advanced nanoparticle
formulation system developed by Inside Therapeutics.
This microfluidic-based platform is designed to support the entire R&D process for RNA-LNP medicines and therapies, from the first screening steps to in-vivo. It incorporates a
reusable chip with an optimized fluidic design that eliminates dead volumes, For those reasons Tamara offers a cost effective solution for RNA-LNP development

TAMARA offer 2 microfluidic mixing approach within one chip that the user can select at will, an optimized Herringbone mixer and a Baffle mixer. Throughout this entire study, the Herringbone mixing approach was employed.
Using Tamara, RNA-LNP formulations have been synthesized under various conditions by adjusting both the Total
Flow Rate (TFR) and Flow Rate Ratio (FRR). Each batch of RNA-LNPs formulated with Tamara has been produced in
volumes of 200, 400, or 700 µL.
This following table recap the formulation condition used:
| Volumes (µL) | TFR (mL/min) | FRR |
|---|---|---|
| 700 | 9,5 | 3:1, 4:1 |
| 400 | 5,4 | 3:1, 4:1 |
| 200 | 4,2 | 3:1, 4:1 |
Note that the same formulation conditions could not necessarily be used at every scale due to the too short
formulation time under low volume/high flow rate conditions.
Notably, the entire study was completed using only two chips—one dedicated to the 700 µL batches and the other for all the 200 and 400 µL batches. Between runs, the chips were thoroughly cleaned using TAMARA’s integrated cleaning function, ensuring consistent performance and repeatability.
IGNITE
Provided by Precision Nanosystems, Ignite is the industry leading nanoparticle formulation system. It features single use cartridges including toroidal mixers for nanoparticle formulation.
In this context, the RNA-LNP formulation had previously been optimized using a DOE approach to determine the
optimal formulation conditions.
All formulation were carried out at 700µL volume.
Post formulation process
Nanoparticles formulated with either system were first diluted into 9 mL of milli-Q water right before being filtered using standard filtration process.
Characterization
RNA-LNP size & PDI
RNA-LNP size & PDI characterization was carried out using a DLS system Zetasizer Ultra Red by Malvern Panalytical. Size corresponds to the z-average for the sample, PDI is the polydispersity index, comprised between 0 and 1, it indicates the level of homogeneity in the LNP sample.
RNA-LNP encapsulation measurement
EE% & EY efficiency was carried out using an adapted RiboGreen assay, and a Clariostar Plus plate reader for
measurement.
EE%, or encapsulation Efficiency, was computed as the amount of RNA encapsulated over the total final amount of RNA.
EY%, or encapsulation Yield, refers to as the amount of RNA encapsulated over the initial RNA.
Cell assays
T -1: Transfection Day -1:
Cells were seeded in the appropriate well plate, such that the cells were at 70-80% confluent on the end point.
T0: Transfection Day:
– Media was removed from the cells
– mRNA-LNP was added at a concentration of max 2 µg/mL of mRNA, 0.5 µg of mRNA per well.
– Cells were incubated with the treatment for 24 hours
Additional details:
o The mRNA-LNP treatment were prepared in Opti-MEM™ I Reduced Serum Medium (a media optimized for
transfection that allows the use of a reduced amount of FBS). When incubated overnight, FBS was added to the
optimem.
Flow cytometry
Post transfection, the protein expression has been assessed by FACS using the cell’s eGFP. Here, the GeoMFI
(Geometric Mean Fluorescence Index) has been used as comparison point to evaluate level of protein expression.
In any event, media was replaced by FACS buffer before before FACS was carried out.
FACS buffer composition: PBS without MgCl2 +5% FBS+ 2.5 mM EDTA+ DNase I 30 µg/mL+ 0.5% sodium azide.
Cytoflex S was used to measure GFP expression, cell death was determined by adding propidium iodide to the cells before passing on FACS.


Looking to get started or improve your LNP formulation screening?
Reach out to us to discover how we can help!
Other Application notes
Looking for more detailed application notes on nanoparticle formulations? Check out our other resources!
See all Application notes
