Abstract
RNA–lipid nanoparticles (RNA–LNPs) have emerged as the leading delivery platform for RNA therapeutics, yet manufacturing consistency across scales remains a major challenge. This article reviews current RNA–LNP formulation and mixing technologies, highlighting their impact on critical quality attributes and scalability. It also introduces NanoPULSE, a scalable micromixing platform designed to bridge early-stage formulation screening and clinical-scale manufacturing, enabling reproducible RNA–LNP production from discovery to the clinic.
Written by the Inside Therapeutics team and first published in ImmunoWatch – Non-Viral Delivery (MabDesign). Link for download available at the bottom of the article.
The rise of RNA-LNPs
The past decade has witnessed RNA-LNP technologies move from concept to clinic, with mRNA vaccines against COVID-19 marking their most transformative milestone. The success of these vaccines has validated LNPs as the leading delivery platform for nucleic acids, offering biocompatibility, versatility, rapid development timelines, and scalability. [1] Beyond infectious diseases, LNP-mediated drug delivery also holds great promises for immunotherapy, oncology, rare diseases, and gene therapy. [2,3] Industry pipelines are expanding at an unprecedented pace, with LNP platforms progressing through all stages of R&D and clinical testing. In this context, achieving consistent particle properties and robust scale-up is a key factor in translating LNP technology from the laboratory to patients.
Within this landscape, this review examines current RNA–LNP manufacturing strategies, outlining their strengths and limitations, highlights the scaling challenges that impede translation, and introduces two platforms from Inside Therapeutics—TAMARA for R&D and NanoPulse for seamless production across all scales.
Manufacturing LNPs
Impact of manufacturing techniques on LNP quality
The final properties of LNPs depend not only on the choice of lipids, RNA, and solvents but critically on the manufacturing approach. Process parameters influence nearly all critical quality attributes (CQAs), including particle size, polydispersity, surface charge, morphology, encapsulation efficiency and yield. These attributes, in turn, determine pharmacokinetics, cellular uptake, biodistribution, toxicity, and ultimately therapeutic efficacy. Even subtle changes in processing might lead to significant variations in these attributes, emphasizing the need for well-controlled and reproducible production. Selecting the optimal formulation strategy, tailored to the intended route of administration and target tissue, is therefore essential for efficient and reliable LNP drug development. [4]
Overview of LNP manufacturing approaches
LNP manufacturing landscape has evolved from early bulk methods, such as thin film hydration and ethanol injection, toward more sophisticated approaches, including microfluidics and impingement jet mixing (Figure 1). While bulk approaches offer a rapid and easy way to start formulating, microfluidic platforms enable screening at small scale, and IJMs deliver the throughput required for clinical supply. Each approach has trade-offs in scalability, reproducibility, and accessibility. Therefore, understanding these techniques and their limitations provides a useful framework for guiding both process development and regulatory strategy.
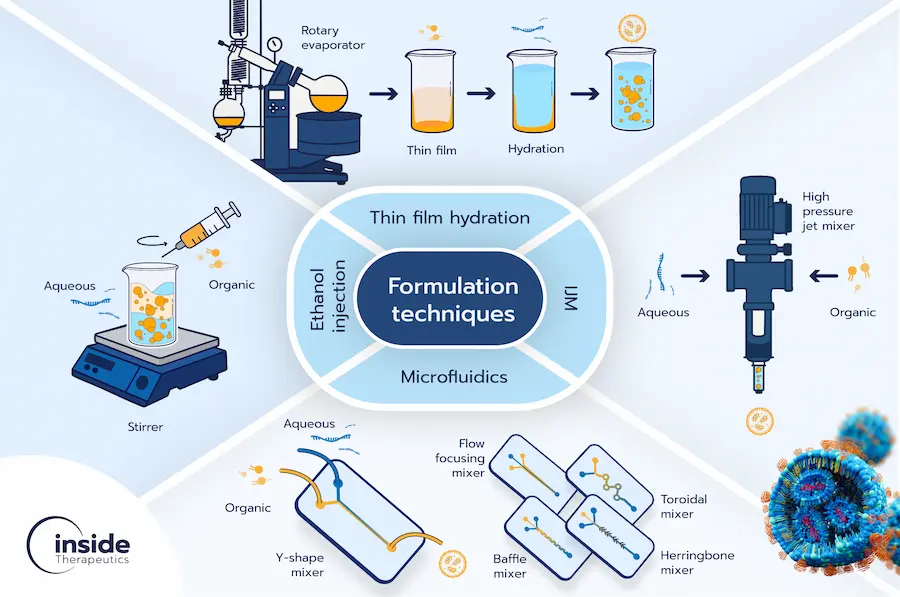
Traditional bulk methods
Early methods for LNP preparation, often referred to as bulk or batch techniques, remain widely used in academic settings. These include thin film hydration and ethanol injection, which provide relatively simple routes for generating nanoparticles.
Thin film hydration is considered the historical starting point for lipid-based nanoparticle production. In this process, lipids are first dissolved in an organic solvent, which is then evaporated to create a thin lipid film on the flask’s wall. Upon hydration with an aqueous phase, the lipids self-assemble into multilamellar vesicles. The resulting particles are usually large (>100 nm) and polydisperse, necessitating additional size reduction steps such as extrusion, high-pressure homogenization (HPH), or sonication. Although practical at small volumes, this method is challenging to scale up and suffers from low encapsulation efficiency and poor reproducibility. [4–6]
On the other hand, ethanol injection involves dissolving lipids in ethanol and injecting the solution into an aqueous phase under stirring, where the sudden dilution of ethanol drives spontaneous formation of vesicles. This approach is conceptually simple and reproducible at small scales, but low encapsulation efficiency, batch variability, and poor scalability limit its use beyond early-stage formulation. [6]
Impingement jet mixers
Impingement jet mixers (IJMs) drive two opposing high-velocity fluid streams into direct collision in a confined mixing zone, producing fast and homogeneous mixing. This near-instantaneous turbulent mixing leads to flash nanoprecipitation, enabling the formation of LNPs in a highly controllable and reproducible manner that is essential for clinical applications. [4] Particle characteristics can be finely tuned by adjusting flow rates and mixing chamber geometries. [4,7] Most importantly, IJMs offer a clear route to scale-up through parallelization, enabling continuous, high-throughput production, and translation from bench to industry. As such, IJMs became the method of choice for large-scale mRNA vaccine production during the COVID-19 pandemic, where companies such as Pfizer implemented parallel IJM systems to achieve continuous, GMP-compliant synthesis. [4,6] Commercially, companies such as Knauer provide IJM platforms at multiple scales. [8] Despite these benefits at industrial volumes, relatively large minimum working volumes can limit their utility in early-stage screening studies.
Microfluidic approaches
Microfluidics has revolutionized RNA–LNP production by enabling tightly controlled nanoprecipitation with far greater reproducibility than bulk methods. In microfluidic devices, LNPs are generated at the aqueous–ethanol interface through diffusion-driven solvent exchange. When lipid-dissolving ethanol is diluted below a critical concentration by the RNA-containing aqueous buffer, spontaneous self-assembly yields small nanoparticles. [9] The microscale dimensions promote rapid mixing, as well as efficient mass and heat transfer, collectively resulting in homogeneous LNP populations. By finely tuning process parameters such as aqueous-to-organic flow rate ratio (FRR) and total flow rate (TFR), researchers can systematically modulate particle size, polydispersity, and encapsulation efficiency [4,10]. This level of reproducibility and control is essential for RNA-LNPs, where subtle physicochemical shifts can dramatically alter biological performance. Microfluidic platforms support high-throughput screening and optimization, accelerating discovery-to-preclinical translation. These advantages position microfluidics as a critical enabler of next-generation LNP therapeutics. In the following subsections, we review the principal micromixer architectures employed for RNA–LNP production—including T- and Y-junctions, hydrodynamic flow focusing, staggered herringbone, and toroidal mixers—highlighting their respective advantages and limitations.
T- or Y-mixer
T- and Y-mixers represent some of the earliest microfluidic platforms developed for LNPs. In these flat microchannel devices, organic and aqueous streams converge at an orthogonal or angled junction, and nanoparticle formation occurs at the interface through solvent diffusion and dilution. Operating typically at high flow rates (40–60 mL/min), these mixers enable production of larger LNP batches but require relatively high material inputs, making it less suited to discovery studies. Their use is hindered by limited control over particle size and throughput volume. These constraints have motivated the development of more advanced microfluidic geometries that enhance size control and accommodate lower-volume applications. [4]
Hydrodynamic flow focusing
By directing an organic lipid solution into a central channel and sheathing it with aqueous buffer streams, hydrodynamic flow focusing (HFF) generates a narrow laminar jet that undergoes rapid diffusion-driven mixing, yielding small (<150 nm) and narrowly distributed nanoparticles. Despite producing uniform RNA–LNPs with tunable sizes, the low throughput of typical chip-based 2D HFF (<10 mL/h) restricts its use beyond small-scale applications, and channel clogging remains a challenge. [4,6] Capillary-based 3D HFF systems have been developed [11], achieving several-fold increases in production capacity, though at the expense of higher device complexity, operational cost, and dilution of formulations. As a result of these limitations, HFF has not achieved the widespread adoption seen with other microfluidic platforms. [4,6]
Staggered herringbone micromixer
Among microfluidic designs, the staggered herringbone micromixer (SHM) has proven particularly powerful for RNA–LNP production. The asymmetric herringbone grooves embedded into the channel floor generate transverse flows that induce chaotic advection, enabling near-instantaneous mixing (<10 ms). This rapid diffusion at the organic–aqueous interface produces highly homogeneous RNA–LNPs with tunable sizes (as small as 30 nm) and excellent reproducibility [4,6], offering greater control than T- or Y-junction mixers at comparable operating flow rates. [9] SHM technology has been widely adopted, including in commercial platforms such as those developed by Precision NanoSystems (now part of Cytiva) [12], or Inside Therapeutics, and has become a standard for preclinical RNA–LNP pipelines. Nevertheless, the relatively low throughput of SHM devices (<100 mL/h) constrains their application to early development, creating a bottleneck when moving toward GMP-scale manufacturing. Recent innovations in parallelization [4,6], alternative geometries (e.g., toroidal mixers) [13], and antifouling surface treatments (e.g., perfluorodecalin coating) [14], aim to overcome this limitation, enabling microfluidic mixing technologies to support higher-throughput manufacturing.
Toroidal mixers
Toroidal (also known as bifurcating) mixers utilize a series of toroidal channels that repeatedly split and recombine fluid streams to generate rapid and chaotic mixing, producing highly uniform LNPs with tight control over size, polydispersity, and encapsulation efficiency. Compared with traditional SHM mixers, toroidal designs support substantially higher total flow rates (up to 200 mL/min). [4,15] This approach preserves compatibility with early-stage and preclinical production and simplifies the transition to larger-scale manufacturing. [5] The NxGen™ by Precision Nanosystems (now part of Cytiva) platform exemplifies the practical use of this design. [4,15]
Baffle mixers
Baffle mixers represent an alternative microfluidic platform for the controlled synthesis of lipid-based nanoparticles. By incorporating a series of perpendicular turns within the microfluidic channels [16], these mixers induce secondary flows, backflow, and recirculation, which enhance the rapid mixing of aqueous and organic streams. This architecture allows precise tuning of LNP size, exemplified by the iLiNP system. [17] Compared with the complex three-dimensional grooved structures of SHM devices, the simpler two-dimensional design of baffle mixers could improve robustness and reduce the risk of channel clogging and flow stagnation. [17]
Case study: Comparing different microfluidic LNP formulation approaches
In this section, we evaluate the performance of the TAMARA formulation system (Inside Therapeutics) relative to toroidal mixers, highlighting its capabilities and limitations for RNA–LNP production.
The TAMARA system enables RNA-LNP synthesis from screening volumes (~0.2 mL) up to in vivo scales (~30 mL). It integrates a dual mixer architecture combining a hydrodynamic flow-focusing junction with either staggered herringbone or baffle structures in a single reusable chip. This hybrid configuration enhances mixing efficiency compared with conventional herringbone-only designs. [18] By controlling critical process parameters, including TFR and FRR, TAMARA allows fine-tuning of nanoparticle size (~50–200 nm), polydispersity index (<0.2), and encapsulation efficiency (>98%), with high batch-to-batch reproducibility (Figure 2).
Head-to-head comparisons between TAMARA and toroidal mixers show comparable encapsulation efficiency, with TAMARA achieving higher encapsulation yield at low working volumes (e.g., >90% at 700 µL versus ~75% for toroidal mixers). In vitro evaluation demonstrated similar transfection efficiency and cell viability across systems, while TAMARA-formulated LNPs exhibited up to 30% higher protein expression (Figure 3).
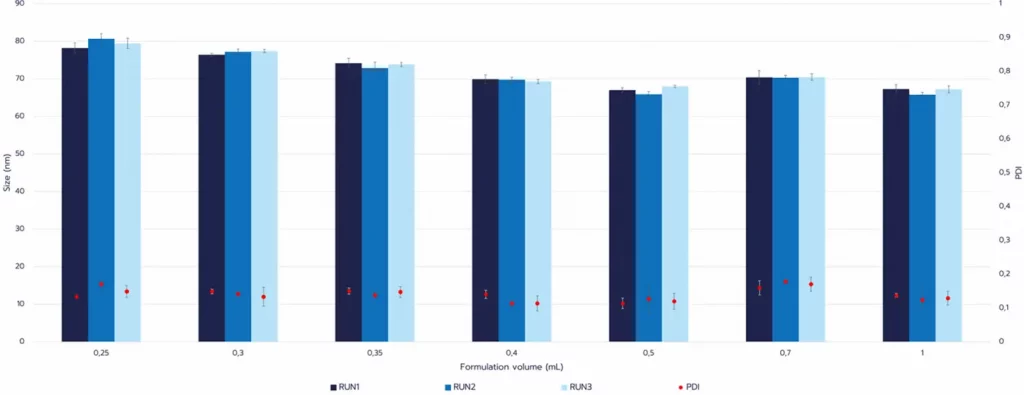
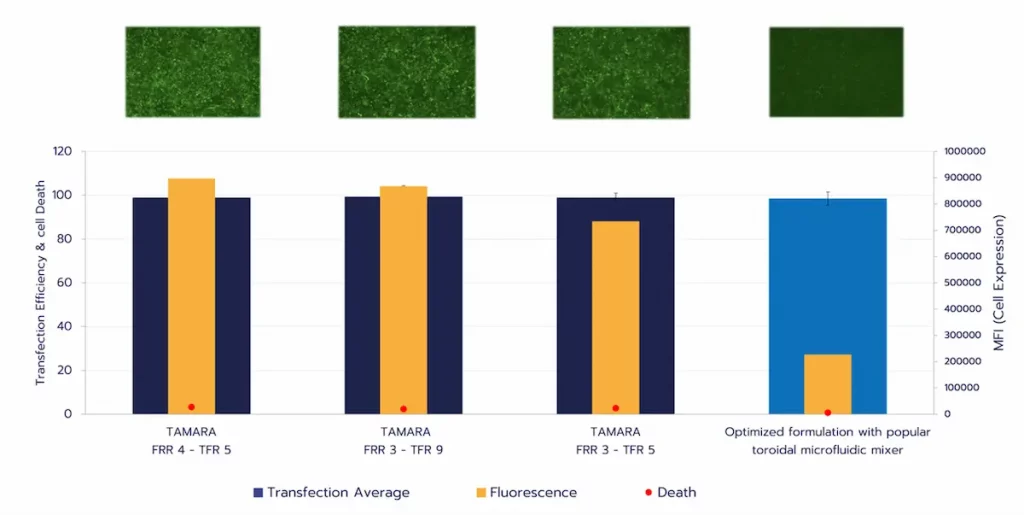
Taken together, these findings illustrate how microfluidic design impacts formulation outcomes. However, throughput limitations remain a key constraint for translation to GMP manufacturing. To place this in context, the next section will discuss scaling challenges across different RNA–LNP manufacturing technologies.
Scaling challenges across manufacturing techniques
The development of RNA-LNP therapeutics is critically constrained by the absence of a single formulation technology that spans the entire pipeline—from microliter-scale discovery to large-scale clinical manufacturing. Microfluidic devices dominate early discovery, yet these systems face throughput and scalability limitations, making them unsuitable for clinical production. Conversely, IJMs are well established for high-volume manufacturing, but they struggle to handle the low-volume formulations needed for early-stage screening. [4,6]
Current workflows typically require transitioning between different mixing platforms as volumes increase, which can alter CQAs of nanoparticles. Each transition necessitates re-optimization and re-validation, slowing down therapeutic development and increasing the risk of failure. A single, scalable technology capable of spanning all scales of production is therefore essential to overcome operational and regulatory challenges. In the next section, we introduce NanoPULSE (Inside Therapeutics), a technology designed to address this gap.
NanoPULSE: A new platform bridging early development and clinical manufacturing workflows
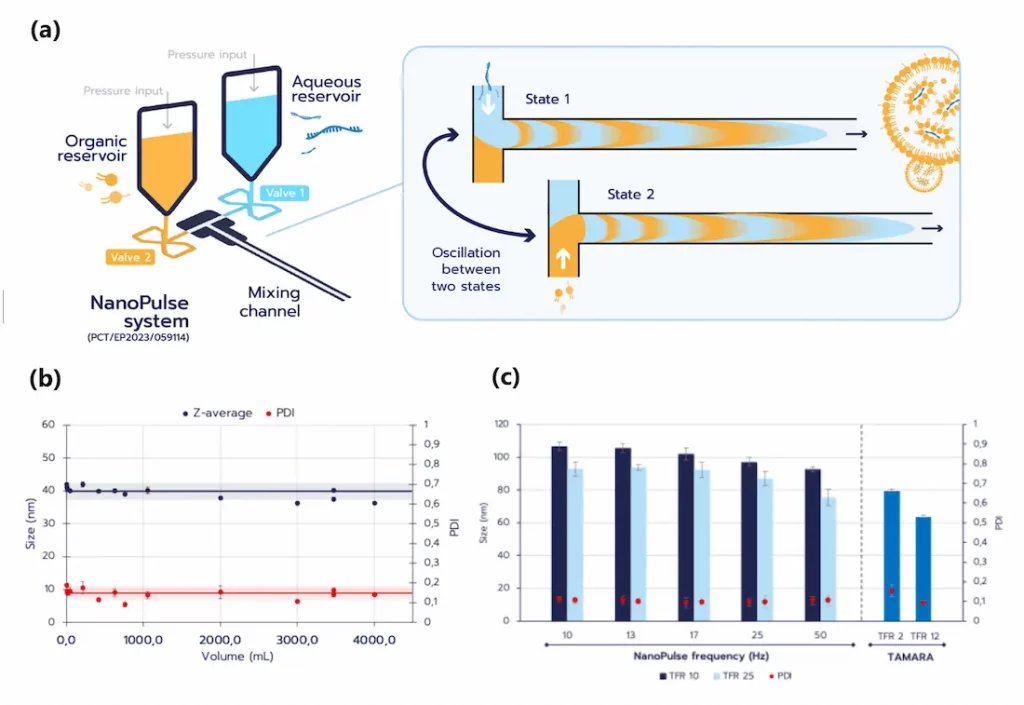
NanoPULSE, a patented micromixing technology developed by Inside Therapeutics, provides a unified platform for RNA-LNP formulation across all scales. It employs high-frequency valve actuation to sequentially inject aqueous and organic solvent fringes into a T-junction. The fringes quickly diffuse into one another through Taylor–Aris dispersion (Figure 4a). This pulse-driven, self-cleaning design enables precise control of mixing dynamics while avoiding aggregation, ensuring reproducible nanoparticle synthesis from microliter-scale screening to continuous tens-of-liters production. Lagrangian simulations, implemented in Python, were used to model how input parameters influence nanoparticle formation and to refine channel geometry. These studies also introduced a novel index to quantitatively assess mixing efficiency, providing a predictive framework for nanoparticle synthesis with NanoPULSE.
The key advantage of NanoPULSE lies in its scalability. Proof-of-concept studies demonstrated continuous liposome manufacturing up to ~40 L/day, with dynamic light scattering (DLS) confirming consistent particle size and polydispersity (<5% variation) from 500 µL to 4 L (Figure 4b). Moreover, tuning the pulse frequency enables modulation of RNA-LNP size (Figure 4c), highlighting platform’s flexibility for diverse therapeutic applications. Encapsulation efficiency and structure of these RNA-LNPs were comparable to those synthesized using TAMARA.
By bridging discovery-scale experimentation with clinical-scale manufacturing, NanoPULSE has the potential to overcome two major bottlenecks in RNA-LNP development: variability induced during scale-up and aggregation during continuous production. Its scalability, reproducibility, and efficiency accelerate RNA-LNP therapeutic development while reducing regulatory complexity during scale-up.
Conclusion
The LNP landscape continues to evolve rapidly, driven by the expanding application of RNA-based therapeutics beyond vaccines. Despite remarkable progress, several challenges remain in the industrial development of RNA-LNPs, including formulation stability, long-term storage, and compliance with regulatory and quality standards. [4] These hurdles are further complicated by the growing demand for continuous and reproducible manufacturing processes capable of maintaining CQAs across scales. Current technologies are lacking in this respect, fragmenting the development process and slowing down the translation process. Consequently, there is an increasing emphasis on robust, flexible, and scalable platforms that can streamline the transition from early discovery to clinical production while satisfying regulatory expectations.
Within this context, microfluidic-based methods represent a reliable preclinical platform, enabling low-volume screening with reproducible nanoparticle characteristics, minimal reagent consumption, and rapid optimization of formulation parameters. In parallel, NanoPULSE seamlessly translates formulation principles from microliter-scale discovery to multi-liter clinical production, supporting the full continuum of RNA-LNP development. By enabling scalable and high-quality production of nanoparticles, NanoPULSE paves the way for the next-generation of RNA therapeutics—including cancer immunotherapies, gene-editing applications, and protein replacement strategies. By reducing the barriers between bench-scale discovery and clinical translation, this technology has the potential to accelerate therapeutic development, streamline regulatory pathways, and ultimately expand the accessibility and impact of RNA-based medicines.

Looking to get started with RNA-LNP formulation?
Reach out to us to learn how we can help!
Acknowledgments
We sincerely thank our esteemed collaborators, notably Chantal Pichon, Milad Baroud and Nabila Laroui at Inserm ART ARNm laboratory, as well as Brice Calvignac and Marie Bonnin at Inserm MINT laboratory. We are also grateful to Julien Ridouard for his creative input and support in designing and organizing the figures.
Also, thank you very much for MabDesign for the opportunity to take part to the immunowatch. Download the complete document on non-viral delivery below.
References
1. Fang E, Liu X, Li M, Zhang Z, Song L, Zhu B, et al. Advances in COVID-19 mRNA vaccine development. Signal Transduct Target Ther. Springer Nature; 2022. https://doi.org/10.1038/s41392-022-00950-y
2. Sahin U, Karikó K, Türeci Ö. MRNA-based therapeutics-developing a new class of drugs. Nat Rev Drug Discov. Nature Publishing Group; 2014. p. 759–80. https://doi.org/10.1038/nrd4278
3. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines-a new era in vaccinology. Nat Rev Drug Discov. Nature Publishing Group; 2018. p. 261–79. https://doi.org/10.1038/nrd.2017.243
4. Mehta M, Bui TA, Yang X, Aksoy Y, Goldys EM, Deng W. Lipid-Based Nanoparticles for Drug/Gene Delivery: An Overview of the Production Techniques and Difficulties Encountered in Their Industrial Development. ACS Materials Au. American Chemical Society; 2023. p. 600–19. https://doi.org/10.1021/acsmaterialsau.3c00032
5. Webb C, Ip S, Bathula N V., Popova P, Soriano SKV, Ly HH, et al. Current Status and Future Perspectives on MRNA Drug Manufacturing. Mol Pharm. American Chemical Society; 2022. p. 1047–58. https://doi.org/10.1021/acs.molpharmaceut.2c00010
6. Xu S, Hu Z, Song F, Xu Y, Han X. Lipid nanoparticles: Composition, formulation, and application. Mol Ther Methods Clin Dev. Cell Press; 2025. https://doi.org/10.1016/j.omtm.2025.101463
7. Devos C, Mukherjee S, Inguva P, Singh S, Wei Y, Mondal S, et al. Impinging jet mixers: A review of their mixing characteristics, performance considerations, and applications. AIChE Journal. John Wiley and Sons Inc; 2025. https://doi.org/10.1002/aic.18595
8. KNAUER Wissenschaftliche Geräte GmbH. Impingement Jets Mixing Systems – from R&D to manufacturing. [Internet]. 2025 [cited 2025 Sep 17]. https://www.knauer.net/lipid-nanoparticle-production. Accessed 17 Sep 2025
9. Bi D, Wilhelmy C, Unthan D, Keil IS, Zhao B, Kolb B, et al. On the Influence of Fabrication Methods and Materials for mRNA-LNP Production: From Size and Morphology to Internal Structure and mRNA Delivery Performance In Vitro and In Vivo. Adv Healthc Mater. John Wiley and Sons Inc; 2024;13. https://doi.org/10.1002/adhm.202401252
10. Roces CB, Lou G, Jain N, Abraham S, Thomas A, Halbert GW, et al. Manufacturing considerations for the development of lipid nanoparticles using microfluidics. Pharmaceutics. MDPI AG; 2020;12:1–19. https://doi.org/10.3390/pharmaceutics12111095
11. Hood RR, Devoe DL, Atencia J, Vreeland WN, Omiatek DM. A facile route to the synthesis of monodisperse nanoscale liposomes using 3D microfluidic hydrodynamic focusing in a concentric capillary array. Lab Chip. Royal Society of Chemistry; 2014;14:2403–9. https://doi.org/10.1039/c4lc00334a
12. Walsh C, Ou K, Belliveau NM, Leaver TJ, Wild AW, Huft J, et al. Microfluidic-based manufacture of siRNA-lipid nanoparticles for therapeutic applications. Methods in Molecular Biology. Humana Press Inc.; 2014. p. 109–20. https://doi.org/10.1007/978-1-4939-0363-4_6
13. Mendonça MCP, Kont A, Kowalski PS, O’Driscoll CM. Design of lipid-based nanoparticles for delivery of therapeutic nucleic acids. Drug Discov Today [Internet]. 2023 [cited 2025 Sep 1];28. https://doi.org/10.1016/j.drudis.2023.103505
14. Hwang YH, Shepherd SJ, Kim D, Mukalel AJ, Mitchell MJ, Issadore DA, et al. Robust, Scalable Microfluidic Manufacturing of RNA-Lipid Nanoparticles Using Immobilized Antifouling Lubricant Coating. ACS Nano. American Chemical Society; 2025;19:1090–102. https://doi.org/10.1021/acsnano.4c12965
15. Osouli-Bostanabad K, Puliga S, Serrano DR, Bucchi A, Halbert G, Lalatsa A. Microfluidic Manufacture of Lipid-Based Nanomedicines. Pharmaceutics. MDPI; 2022. https://doi.org/10.3390/pharmaceutics14091940
16. Osouli-Bostanabad K, Puliga S, Serrano DR, Bucchi A, Halbert G, Lalatsa A. Microfluidic Manufacture of Lipid-Based Nanomedicines. Pharmaceutics. MDPI; 2022. https://doi.org/10.3390/pharmaceutics14091940
17. Kimura N, Maeki M, Sato Y, Note Y, Ishida A, Tani H, et al. Development of the iLiNP Device: Fine Tuning the Lipid Nanoparticle Size within 10 nm for Drug Delivery. ACS Omega. American Chemical Society; 2018;3:5044–51. https://doi.org/10.1021/acsomega.8b00341
18. Kozalak G, Heyat Davoudian S, Natsaridis E, Gogniat N, Koşar A, Tagit O. Optimization of PLGA Nanoparticle Formulation via Microfluidic and Batch Nanoprecipitation Techniques. Micromachines (Basel). Multidisciplinary Digital Publishing Institute (MDPI); 2025;16. https://doi.org/10.3390/mi16090972