Abstract
Modern nanomedicine leverages lipid-based nanoparticles for cancer therapies to achieve precise drug delivery. The article details passive and active targeting mechanisms, PEGylation, and antibody conjugation, highlighting how these design strategies improve biodistribution, stability, and therapeutic performance in oncology.
In the ever-evolving landscape of cancer-therapies, the convergence of cutting-edge nanoparticles technology and health innovations has brought forth a promising paradigm shift. The use of lipid-based nanoparticle, such as albumin or liposomes, for cancer therapies has recently been brought to the spotlight for their innovative way of accessing cancerous tissues. Come along with us on this exploration to discover the distinct features of tumor cells and the delivery of drugs via nanoparticles.
Tumoral cell specificities exploited for lipid-based nanoparticle cancer therapies
A tumoral cell is characterized by the loss of balance between intracellular signals. The tumoral cell is silencing the inhibitory signals and enhancing the stimulation signals, which leads to uncontrolled cancer growth. This leads to major modifications of the cell behavior:
On a tissular level, here are some noteworthy changes: there is an unstoppable replication, a loss of function, the cell’s immortality, and angiogenesis. Angiogenesis is an abnormal growth of blood vessels which slows down the local blood circulation. This leads to a reduced efficiency of the blood-circulation-dependent treatments at classic efficient-dose, therefore requiring an increased dosing, which can be problematic as it also leads to possible toxicity.
On a membranal level, tumoral cells are observed to have variations in the number of surface receptors (changing cell sensitivity to the regulating mechanisms of the host), and the loss of basal membrane. These two factors can reduce the impact of receptors dependent treatments.
Why using lipid-based nanoparticle for cancer therapies instead of other administration routes and other galenic?
Common cancer treatments encompass surgical resection, chemotherapy, radiotherapy, and biological therapy. Surgery is highly effective in removing malignant solid tumors. Combined therapy involves a coordinated use of surgery, chemotherapy, and radiotherapy. Chemotherapy’s indiscriminate cytotoxicity often results in undesired side effects. Thus, challenges faced by current chemotherapy include lack of specificity, cytotoxicity, short half-life, poor solubility, multi-drug resistance, and the growth of stem-like cells.
Therefore, the need for more efficient and less toxic cancer treatment solution appear critical both to increase the success rate and patient’s comfort.
An appealing alternative: nanoparticles
The use of nanoparticles, to deliver therapeutics appears as an alternative solution to help meet those requirements. Let’s briefly review the cellular changes that lipid-based nanoparticles can exploit for cancer treatments delivery.
First, its ability to encapsulation of the active agents within a shell in nanoparticles will increase their solubility and biocompatibility, their stability in bodily fluids and retention time in the tumor vasculature. Additionally, nanoparticles can be engineered to be extremely selective for a precise target, helping to increase their efficiency. [2] Moreover, encapsulating anticancer drugs in liposomes or LNPs reduces tissue toxicity and the rate of drug metabolism, controls drug release and extends circulation time of drugs.
Furthermore, nanoparticle have also opened the door for the development of novel anti-cancer therapies, thanks to their unique ability to deliver genetic material directly into the cells.
Let’s now have a closer look at the cellular mechanisms induced by lipidic-nanoparticles in the tumor microenvironment.
Action mechanism of lipid-based nanoparticle for cancer therapies: cellular level
As previously mentioned, lipidic nanoparticles help enhance drug delivery to tumors, reducing the side effects of traditional therapies. First, we can notice a change in pH cell around the tumor. Lipidic nanoparticles have a pH-responsive behavior: they can switch from a zwitterionic (neutral form) to a cationic charge significantly improving cellular uptake, thus, optimizing its therapeutic potential. [4]
Within the Tumor MicroEnvironment (TME), lipid-based nanoparticles induce apoptosis in cancer cells through various mechanisms. [3]
After discussing the details of lipid-based nanoparticles as a cancer treatment, let’s now explore strategies for specifically targeting these cancerous cells.
Optimizing performances of lipid-based nanoparticle for cancer therapies
Other nanoparticles attributes such as size can also be tuned to improve their cell specificity. Two main nanoparticle targetings strategies are available: passive and active targeting.
Targeting strategies with lipid-based nanoparticle for cancer therapies
Passive targeting
Tumoral cells have some characteristics that are used by pharmaceutics to destroy them: in fact, the endothelial cells permeability in tumoral tissues is higher, and the retention of the cells is enhanced too: it’s the EPR effect.
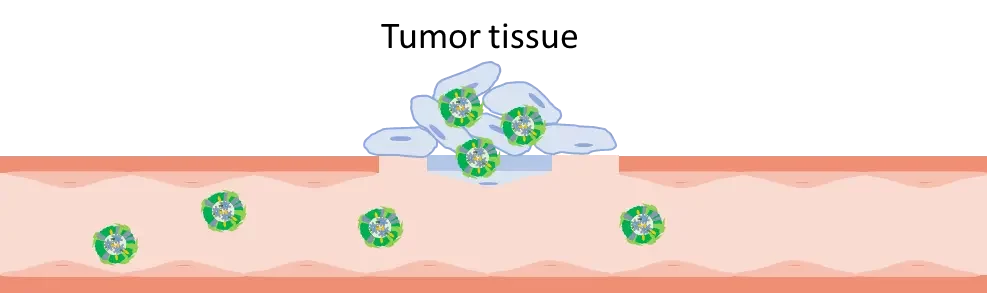
Thus, the first way to target tumoral-cells with mRNA-LNP is passive: by injecting the nanoparticles directly into the tumor site, the mRNA-LNP acts as an immune-stimulating cell and leads to an antitumor immune response by the tumor microenvironment (TME). This TME activation produces cytokines at a local level and prevents toxicity that couldn’t be avoided on a systemic level. This method has its own limits, such as the accessibility of the tumor and the patient tolerability of potential expression outside the cancer cells.
Active targeting strategy: Antibodies
LNP formulation: adding lipidic anchor
Cancer targeting starts during the LNP formulation: a lipidic “anchor” can be added directly in the formula and allows a later conjugation with a functional ligand such as antibodies. However, there is a major risk in using this method: Fc regions of the antibodies can be exposed, leading to unwanted immune reactions. Thus, this strategy needs cleaning steps and generates a lot of waste.
LNP post-formulation: post-insertion of hydrophobic antibody derivate
With this method, preformed LNPs are co-incubated with a hydrophobic antibody derivate. No lipidic anchor is needed in the formula, and therefore, the conjugation process is well-controlled. Moreover, the physicochemical properties of LNPs remain unchanged.
As described in the literature, post-inserted hydrophobic antibodies derived into LNPs for mRNA delivery in cancer cells of mice came back successfully.
Endogenous targeting: choice of the right formula
Using tissue location, cancer cells are targeted by tweaking formulations: as explained in our review about LNP optimization, the cellular biodistribution of LNPs relies on its lipid composition. Thus, using different PEG-linked lipid percentages in LNPs is an efficient way to target different tissue locations and finally to achieve various cellular responses.
Here are some organs that could be targeted:
| Tissue location | Liver | Lymphoid organs |
|---|---|---|
| Targets | – Hepatocellular carcinomas – ↑ Production of antitumoral protein | – Leukocytes and their antitumor function |
Clinical applications examples using lipid-based nanocarriers
Here is a shortlist of some clinic studies using lipid-based nanoparticle for cancer treatment delivery. [8]
Doxil
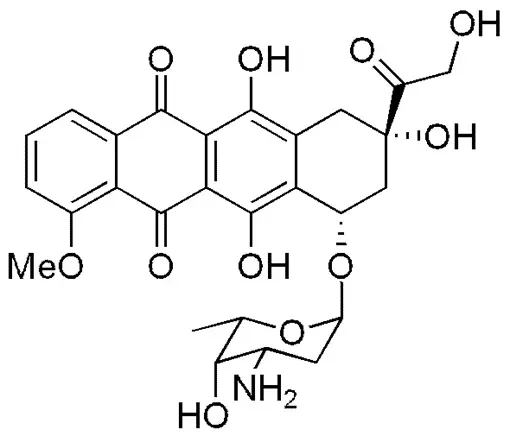
In 1995, Doxil, a liposomal formulation of doxorubicin was created.
In fact, the necessity for developing a liposomal formulation with fewer side effects arose due to the side effects associated with conventional doxorubicin therapy. Utilizing liposomes as a delivery system offers several advantages.
Advantages
Notably, the phospholipids used to create these vesicles are sourced from natural substances like egg yolks or soybeans, ensuring their safety within the body. Moreover, the drug release rate can be adjusted by modifying the degree of saturation in the phospholipid bilayer. The mechanism of action relies on the liposomes’ inability to escape vascular areas with tight capillary junctions, like the heart muscle, while allowing them to exit the circulation in tissues and organs where cell junctions are not tightly bound, such as tumor cells. This leads to a preferential accumulation of anthracyclines in tumor tissue, reducing exposure in areas commonly associated with conventional anthracycline-related toxicity, like the myocardium. Doxorubicin functions by interacting with DNA through intercalation, thereby inhibiting macromolecular biosynthesis. [6]
Abraxane
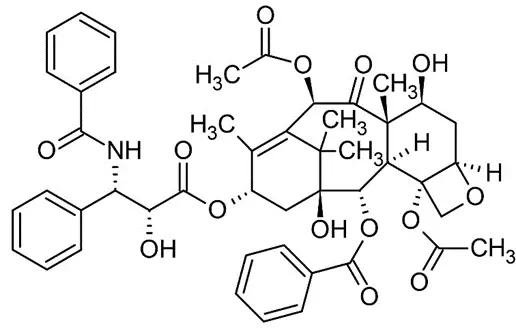
In 2005, Abraxane, a nanoparticle-bound albumin formulation of paclitaxel[9], was introduced. In the formulation, the drug is mixed with the aqueous solution of HSA, and then the mixture is passed through a high-pressure jet to form the drug-albumin nanoparticles. These nanoparticles showed a size of 130 nm. This formulation, named nab-paclitaxel, was approved by the FDA, under the trade name of Abraxane®, for treatment of metastatic breast cancer.
Action mechanism
The action mechanism study makes an echo with the endogenous targeting previously discussed. This shown that albumin can bind to special receptors overexpressed on cancer cells and enhance nanoparticles binding and internalization. The binding to the endothelium was enhanced by 9.9 folds, and paclitaxel was transported more efficiently, compared to the non-nanoparticle one. [7]
Conclusion
To conclude, in the ever-evolving field of cancer therapy, lipid-based nanoparticles offer a promising approach. They enhance drug solubility, biocompatibility, and target specificity, improving treatment effectiveness while reducing toxicities. Clinical studies highlight their potential for more efficient and targeted cancer treatment, representing a significant advancement in the field.

Looking for the right LNP formulation solution?
Reach out to us to learn how we can help!
References
[1] Tenchov, Rumiana et al. “PEGylated Lipid Nanoparticle Formulations: Immunological Safety and Efficiency Perspective.” Bioconjugate chemistry vol. 34,6 (2023): 941-960. doi:10.1021/acs.bioconjchem.3c00174
[2] Pucci Carlotta, Martinelli Chiara, Ciofani Gianni (2019) Innovative approaches for cancer treatment: current perspectives and new challenges ecancer 13 961
[3] Mundekkad, Deepa, and William C Cho. “Nanoparticles in Clinical Translation for Cancer Therapy.” International journal of molecular sciences vol. 23,3 1685. 1 Feb. 2022, doi:10.3390/ijms23031685
[4] Mundekkad, Deepa, and William C Cho. “Nanoparticles in Clinical Translation for Cancer Therapy.” International journal of molecular sciences vol. 23,3 1685. 1 Feb. 2022, doi:10.3390/ijms23031685
[5] https://www.cancer.gov/nano/cancer-nanotechnology/current-treatments
[6] Rivankar, Sangeeta. An overview of doxorubicin formulations in cancer therapy. Journal of Cancer Research and Therapeutics 10(4):p 853-858, Oct–Dec 2014. | DOI: 10.4103/0973-1482.139267
[7] Hassanin, Islam, and Ahmed Elzoghby. “Albumin-based nanoparticles: a promising strategy to overcome cancer drug resistance.” Cancer drug resistance (Alhambra, Calif.) vol. 3,4 930-946. 3 Nov. 2020, doi:10.20517/cdr.2020.68
[8] “File:Doxorubicin Structure.png.” Wikimedia Commons. 4 oct. 2020, 11:55 UTC. 27 sept. 2023, 14:41 <https://commons.wikimedia.org/w/index.php?title=File:Doxorubicin_Structure.png&oldid=480514963>.
[9] File:Taxol.svg.” Wikimedia Commons. 3 janv. 2023, 11:32 UTC. 27 sept. 2023, 14:45 <https://commons.wikimedia.org/w/index.php?title=File:Taxol.svg&oldid=722435048>.
[10] https://commons.wikimedia.org/wiki/File:Doxorubicin_Structure.png?uselang=fr