Abstract
This review explore the potential of lipid nanoparticles (LNPs, liposomes, SLNs, and NLCs) for drug delivery, exploring their structure, key characteristics (size, charge, encapsulation, PDI), and state-of-the-art manufacturing methods, especially microfluidics. It highlights applications across hydrophobic, hydrophilic and RNA/genetic drugs
In the ever-evolving landscape of modern medicine, researchers are constantly seeking innovative strategies to enhance the efficiency and precision of lipid nanoparticle drug delivery. One of the most promising breakthroughs in this field is the use of lipid-based nanoparticles for drug delivery. These versatile lipidic carriers are transforming the way drugs are administered, offering increased efficiency, improved targeting, and reduced side effects. In this article, we’ll delve into the fascinating world of lipid nanoparticles and their pivotal role in advancing drug delivery, with a particular focus on the delivery of hydrophobic drugs, hydrophilic drugs, and RNA.
Types of lipid nanoparticles for drug delivery
To start with, it is important to clearly differentiate the various types of lipid-based nanoparticles and their respective properties.
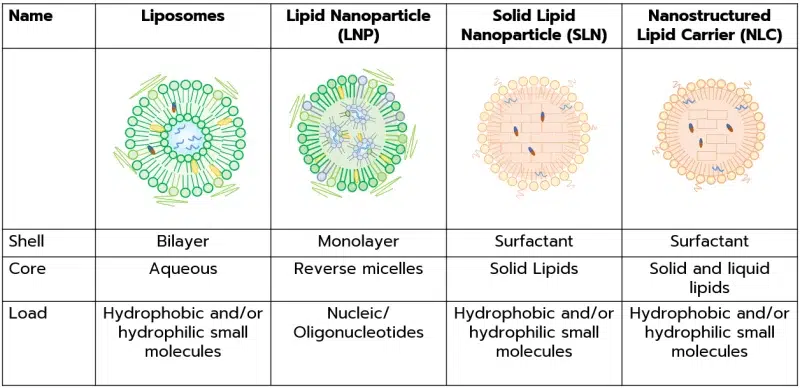
Liposomes are the first lipidic nanoparticle identified in the 1960s. They are characterized by one or more lipid bilayer encasing an aqueous core. They can be further categorized based on lamellarity and size, and can be used for both hydrophilic and hydrophobic payload. You can read more on those in our review on liposomes.
Solid lipid nanoparticles (SLN) feature a surfactant shell surrounding a core matrix composed of solid lipids. Solid lipid nanoparticles are instrumental in encapsulating cargo with either hydrophobic or hydrophilic properties.
Lipid nanoparticles (LNP) consist of a lipid shell that envelops an internal core comprised of reverse micelles. This special kind of nanoparticle has been specifically engineered for the delivery of various nucleic acids, such as siRNA, mRNA, and plasmid DNA. LNP formulation typically consists of 4 lipids: PEG, phospholipid, a sterol, and an ionizable lipid.
Nanostructured lipid carriers (NLCs) comprise a surfactant shell encasing a core matrix composed of both solid and liquid lipids. These carriers are employed for encapsulating cargo with diverse characteristics, including hydrophobic and/or hydrophilic substances.
Lipid nanoparticle main characteristics
Good control of lipid nanoparticle characteristics is critical to achieve successful delivery of their payload to the cells. Here is a list of the most important characteristics to take into account when designing a lipid nanoparticle carrier:
Nanoparticle composition is key as it characterizes its internal structure, and thus its type, impacting the possible cargo types, while affecting its biocompatibility, and delivery efficiency.
Zeta potential corresponds to the measure of the electrostatic field near the nanoparticle surface, therefore affecting its stability. A good balance between a too-neutral (thus leading to agglomeration and instability) and too large (thus leading to toxicity) charge should be kept. Typical zeta potential values are in the order of -30 mv.
Polydispersity (PDI) is a measure of the homogeneity of the sample. Corresponding to the normalized value of the nanoparticle size, it should remain below 0.2. Polydispersity impacts the toxicity and is highly manufacturing process dependant.
Nanoparticle size has a major impact on delivery efficiency and biodistribution. Indeed, the endocytosis process through which the nanoparticles will be internalized depends their size. Typical target size will thus depend on the target cell: For instance, LNP target size is around 80 nm for rodent cells, 60 for primates, and 50 for cancer cells. Learn more about it on our nanoparticle targeting review.
Overall, too large nanoparticle will decrease their delivery efficiency, while too small ones will greatly increase their toxicity.
Encapsulation efficiency is the ratio of API that has been encapsulated over the initial injected amount. It can reach 95% with the most advanced synthesis methods, such as microfluidics.
Manufacturing process
As introduced above, the size, PDI, and encapsulation efficiency of these nanoparticle delivery systems are highly dependent on the manufacturing methods, let’s further explore them: They can generally be categorized into 4 categories:
High energy methods can be used either by themselves to synthesize nanoparticles, or complementary to other methods to further reduce the nanoparticle size. Overall, the goal of these methods is to provide sufficient energy to the system to fragment the nanoparticles. Typical high-energy methods are high-pressure homogenization or ultrasonic homogenization. Overall, they are a cost-efficient method, but offer very poor control over the final nanoparticle characteristics.
Impingement jet mixing/T-junction: To improve the efficiency of nanoprecipitation, impingement jet mixers, and more generally T-mixers, have been developed. They are based on flash nanoprecipitation, where the 2 phases are mixed at very high speed and mix nearly instantly. While being efficient for large batches, those methods require large volumes of liquids and are thus not suitable for the discovery phase. Additionally, the large pressure involved can lead to API degradation.
Batch methods are generally based on nanoprecipitation, where a solvent, containing the lipids, is mixed together with an antisolvent, triggering the self-assembly of the nanoparticles. Batch methods such as thin film hydration consist of mixing those 2 phases in bulk. While this method works, due to the importance of the mixing parameters on lipid nanoparticle size, the control of the final nanoparticle characteristics produced with this method is very poor.
Microfluidics is currently considered as the state-of-the-art manufacturing method for nanoparticles, thanks to its unique ability to carefully control the mixing condition, at both large and low volumes. Learn more on LNP manufacturing process in our review!

Batch Methods | 
High Energy | 
Macrofluidics / T-junctions | 
Microfluidics | |
|---|---|---|---|---|
| Size control | Poor | Poor | Average | Excellent |
| Homogeneity | Low (PDI ~0.5) | Low (multi steps) | Average | Great (PDI <0.2) |
| Encapsulation efficiency | Low | Poor | Good | Excellent (>95%) |
| Repeatability | Low | Low | Low | Great |
| Achievable volumes | μL/mL | μL/mL | mL/L | μL/mL/L |
| Commercial available | Yes | Yes | Yes | Yes |
| Main characteristics | + Affordable – Poor NP control |
– Complex / Expensive – Payload alteration | + Good scalability – Repeatability / NP control | + Best NP control / Scalability – Solvent |
Lipid nanoparticle to encapsulate drugs
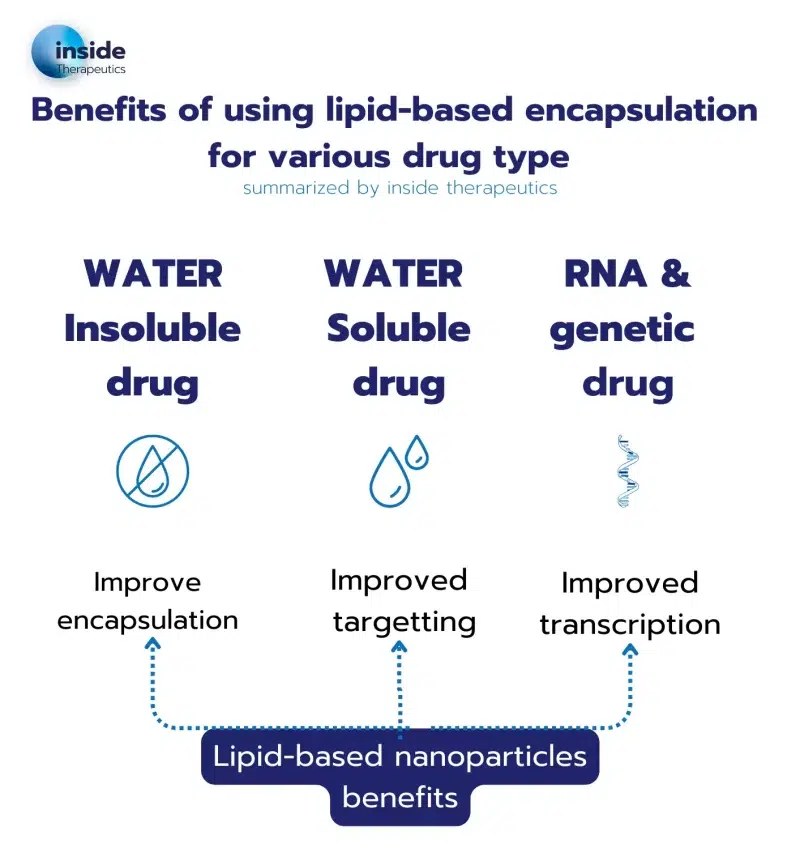
Water-Insoluble Drugs
Around 40% of approved drugs and 90% of drugs in development are not water-soluble. As they are by nature not soluble in water, there therefore a major need to find a suitable carrier for an efficient, and ideally targetted, delivery within the body. A great number of hydrophobic drugs, such as Docetaxel, tretinoin or Cisplatin, have already been delivered using lipid-based A great number of hydrophobic drugs, such as Docetaxel, tretinoin or Cisplatin, have already been delivered using lipid-based nanoparticles. Depending on their types, drugs will either get trapped in the membrane (liposome) or in the core of the nanoparticle (Solid lipid nanoparticle)
Water-Soluble Drugs
Other types of drugs easily dissolve in water, which makes it tricky to control their release. For those, nanoparticles provide an optimal controlled delivery system. Most often, liposomes are used for water-soluble drugs: In this case the hydrophilic drugs are trapped in the core of the liposome, for a more controlled delivery.
RNA and genetic material
The lipid nanoparticle-mediated delivery of nucleic acid, and more specifically of mRNA delivery, has been brought to the spotlight with the Covid 19 vaccine.
For efficient delivery of RNA, older forms of nanoparticles such as liposomes were not suitable because of their instability, poor encapsulation efficiency and difficulties in penetrating the cells. For this reason, a special type of nanoparticle has been engineered: the LNP. LNP’s key component is an ionizable lipid, which has the ability to change charge depending on the pH. This ionizable cationic lipid offers the capability to optimize the RNA encapsulation and delivery (when positively charged), while minimizing toxicity during transit through the body (when neutral). This example perfectly illustrates why tailoring the lipid nanoparticle formulation to the API of interest is critical for an optimal drug release. The range of applications for RNA-LNP is immense, spanning from RNA vaccine for infectious diseases, to cancer vaccines and gene therapies.
Clinical Applications of Lipid Nanoparticle Drug Delivery
The potential applications of lipid nanoparticles as a drug delivery system are vast and rapidly expanding. Some notable examples include:
Infectious disease:
As shown with the Covid 19 vaccines, RNA-LNP therapies has the potential to offer efficient protection against a wide variety of infectious diseases. In this context, the mRNA strand introduced into the cell triggers the production of a protein of the virus surface, helping the immune system recognizing it and produce the corresponding antibody. This versatility has opened doors for the treatment of a wide variety of diseases such as flu, CMV and HIV…
Neurological Disorders:
These nanoparticles can cross the blood-brain barrier, enabling the delivery of drugs to treat neurological diseases such as Alzheimer’s and Parkinson’s.
Cancer Therapy:
Lipid nanoparticles are also utilized to deliver chemotherapeutic agents directly to cancer cells, minimizing harm to healthy tissue and reducing side effects. LNP-mRNA complex can also be used for cancer therapies, either by themselves or in combination with other drugs, by triggering the synthesis of proteins specifically designed to train the immune system to identify and fight cancer cells.
Genetic Therapies: Lipid nanoparticle are used in gene therapy to deliver therapeutic genes or RNA molecules for the treatment of genetic disorders. A typical example is the intracellular delivery of a siRNA strand or an ASO, suppressing the expression of certain genes.
Conclusion on the use of lipid nanoparticle for drug delivery
Lipid nanoparticles have emerged as a transformative force in the field of drug delivery. Their adaptability, precision, and safety make them invaluable tools for delivering a wide range of therapeutic agents. With the advent of mRNA-based therapies , the potential for lipid nanoparticle drug delivery is boundless. As researchers continue to unlock their full potential, these tiny carriers are poised to revolutionize medicine, offering hope for more effective treatments and personalized therapies across a spectrum of diseases. The future of drug delivery revolves around lipid nanoparticles, and the possibilities they hold are truly awe-inspiring.

Looking to improve your LNP formulation process?
Reach out to us to learn how we can help!