Abstract
This guide explains how lipid nanoparticle (LNP) composition critically shapes mRNA Lipid Nanoparticle vaccine and RNA based therapy performance. It outlines the roles of ionizable lipids, helper lipids (phospholipids, cholesterol), and PEG-lipids in encapsulation, stability, and delivery. It also explores formulation parameters and innovative alternatives to enhance targeting and reduce immune interference.
Lipid nanoparticles (LNPs) have emerged as a revolutionary tool in the biotechnology field, particularly for the development of mRNA lipid nanoparticle vaccines (mRNA-LNP) and RNA-based therapies, as they are key for the encapsulation, protection, and delivery of nucleic acid to the target organ and cell. Unlike viral vector delivery methods, which often rely on modified viruses to transport genetic material, LNPs offer a non-viral pathway that significantly enhances the delivery efficiency and safety profile of RNA therapies, as illustrated by the success of the COVID 19 vaccines.
Considering that the lipid composition in LNPs is not a mere formulation detail but a critical aspect that determines the efficacy, safety, and overall success of RNA-based therapies, having an excellent understanding of their composition is key for the eventual success of the therapy.
This article focuses on the lipid composition of RNA-LNP vaccines, starting with a broad introduction to LNP formulation followed by a focus on those that are commercially available and their significance in enhancing the success of RNA therapies.
Overview of lipid nanoparticles composition in vaccines
The choice of lipid nanoparticle for RNA vaccine development is a delicate balance, impacting both biodistribution, delivery target, and encapsulation efficiency. The interaction of various lipids within these vaccines with the human body significantly influences the incidence of side effects, such as localized inflammation or discomfort. While the incorporation of ionizable lipids in current RNA vaccines has enhanced biocompatibility compared to earlier generations of lipid-based nanoparticles, the quest for refined control over their physicochemical attributes, particularly in the context of large-scale production, remains a formidable challenge.
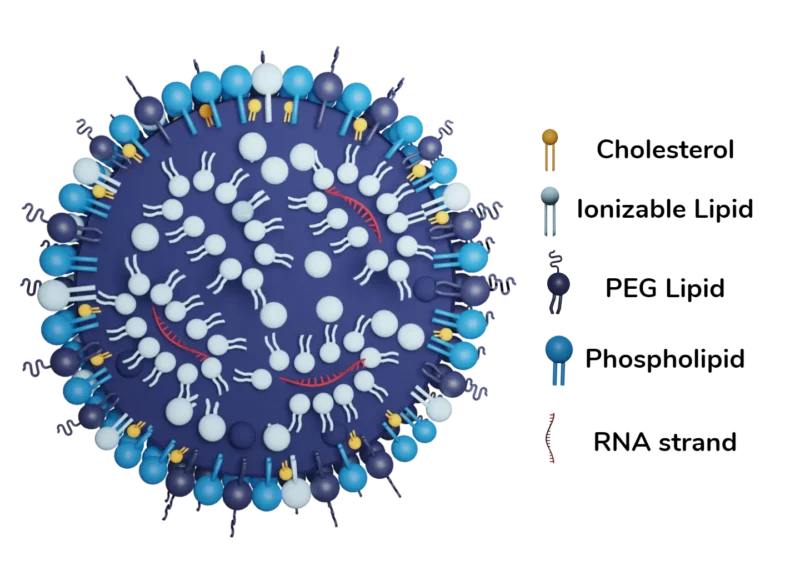
The lipid architecture of FDA-approved RNA vaccines is typically characterized by 4 lipid types: phospholipids, ionizable lipids, sterol lipids, and PEGylated lipids. Each lipid type is carefully selected for its specific role in encapsulating oligonucleotides within an aqueous milieu.
Ionizable Lipids: The Cornerstone of LNP Formulation
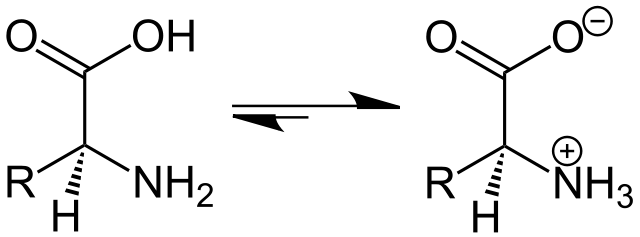
Ionizable lipids are pivotal, constituting approximately 50% of the lipid makeup in LNPs. Thanks to their unique capability to change charge with pH, they perform several critical functions:
- They facilitate efficient encapsulation of RNA during the manufacturing process.
- They enhance the delivery of nucleic acids into the cytosol.
- At a neutral pH, their non-charged state minimizes nanoparticle toxicity.
These lipids form the fundamental core of the LNP, securing the nucleic acid payload. They also contribute to the membrane’s functionality, wherein their charge alteration within the cytosol prompts the structural disruption of the LNP, leading to the delivery of the cargo to the cell.
As introduced below in the detailed vaccine composition, the most widely used Ionizable cationic lipid is the ALC-0315 patented by Acuitas Therapeutics.
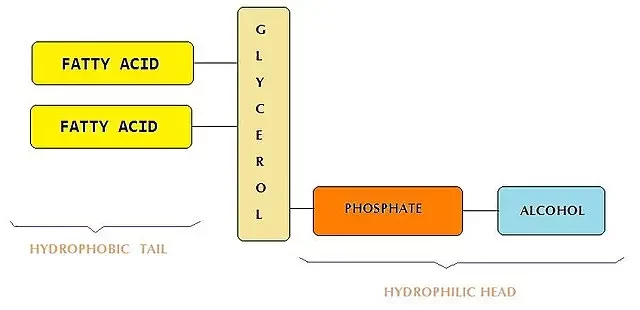
Phospholipids: From Predominance to Precision
Originally the primary component in lipid-based nanoparticles, phospholipids now represent around 10% of the constituent molecules in commercial LNP formulations. Present predominantly in the membrane structure, these lipids are instrumental in augmenting encapsulation efficiency.
Sterol Lipids: The Adaptive Auxiliary
Sterol lipids, primarily cholesterol, act as auxiliary agents. Their functional role is contingent upon the specific lipid they are paired with. In conjunction with phospholipids, sterol lipids confer rigidity and stability to the membrane, facilitating the stable encapsulation of the therapeutic agent and enhancing its longevity and efficacy.
PEGylated Lipids (PEG): A Minor but Crucial Component
Although it constitutes only the smallest fraction of the lipid-based nanoparticle (LNP) composition, the PEGylated lipid has a major impact on several key characteristics, including particle size, aggregation behavior, toxicity, stability, and encapsulation efficacy.
Typically, PEG arranges itself on the lipid carrier’s surface, and its extended tail helps in holding the nanoparticle components together. As a result, even a modest presence of PEG is significant in preserving the LNPs’ optimal size, thereby improving the duration of the systemic circulation and the overall effectiveness of the drug delivery process. Outside concentration, the most critical factors affecting these outcomes are the molar proportion of PEG in the composition and the size of both the lipid tail and the lipid PEG chain.
In summary, lipid nanoparticles (LNPs) used in RNA vaccines consist of a mix of ionizable lipids, phospholipids, sterol lipids, and PEGylated lipids. Each lipid type is critical for optimizing vaccine delivery and efficacy while reducing toxicity. The precise balance among these components is thus essential and must be tailored to the specific RNA cargo and the target cell or organ to achieve optimal results.
Why the Choice of Lipids Is Critical for Developing Successful Drug Products
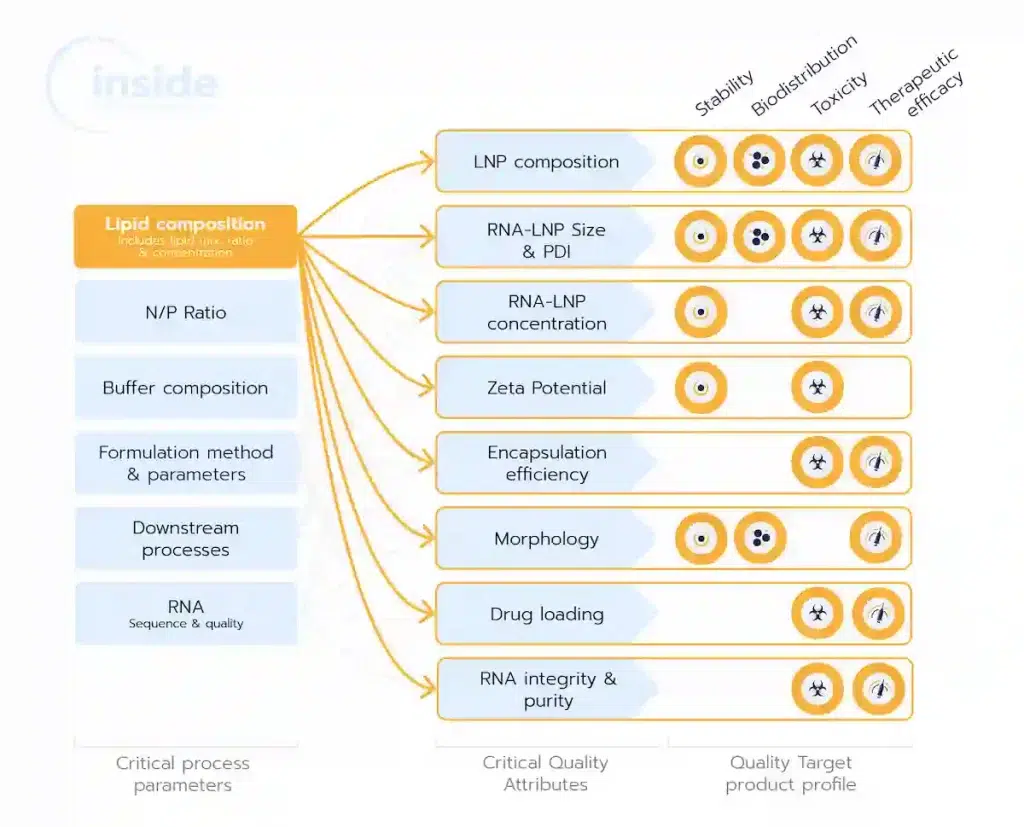
The selection of lipids is a key element in the development of lipid nanoparticles (LNPs) as it significantly impacts their Critical Quality Attributes (CQAs), such as particle size, encapsulation efficiency, stability, and drug release profile. These CQAs, in turn, dictate the pharmacokinetics, efficacy, safety, and overall performance of the drug product.
Each lipid component—whether ionizable lipids for encapsulation, helper lipids for structural stability, cholesterol for membrane integrity, or PEG-lipids for circulation time—plays a specific role in optimizing the LNP formulation. The choice of lipid type, ratios, concentration and purity can affect the nanoparticle’s behavior during formulation, its interaction with biological systems, and its scalability for manufacturing.
A strategic and well-informed lipid selection minimizes risks such as low encapsulation efficiency, poor stability, or toxicity, while ensuring consistent and high-performing LNPs. The right choice of lipids is therefore critical for addressing regulatory requirements, achieving clinical success, and scaling from development to commercial production without compromising quality.
FDA-Approved RNA-LNP Therapies
The landscape of FDA-approved RNA-LNP therapies has been expanding, primarily due to the success of lipid nanoparticles in delivering RNA molecules effectively to target cells. To date, 3 RNA-LNP therapies have been approved :
- ONPATTRO (Alnylam Pharmaceuticals Patisiran): The first RNA-LNP therapy available, this groundbreaking therapy targets the RNA involved in transthyretin-mediated amyloidosis, a rare condition using siRNA-LNP.
- Comirnaty (Pfizer-BioNTech COVID-19 Vaccine): This mRNA vaccine represents a milestone in vaccine technology and public health. Developed using LNP technology to deliver mRNA, Comirnaty instructs cells to produce a protein that triggers an immune response, providing protection against COVID-19.
- Spikevac (Moderna’s mRNA-1273): Similarly, Moderna’s vaccine utilizes LNP-encapsulated mRNA technology. It has been a critical tool in combating COVID-19, highlighting the versatility and potential of RNA-LNP platforms in rapid mRNA vaccine development and deployment during global health crises.
What are the lipid nanoparticle compositions in FDA-approved vaccines?
LNP Composition in the ONPATTRO Vaccine by Alnylam
ONPATTRO (Patisiran) represents a significant breakthrough in RNA interference (RNAi) therapy as it was the first FDA-approved RNA-LNP therapy. This development first demonstrated the vast potential of RNA-based therapeutics and paved the way for future development.
Conceived by Alnylam Pharmaceuticals, this innovative treatment employs lipid nanoparticles to deliver small interfering RNA (siRNA) directly to the liver, drastically reducing harmful TTR protein production.
siRNA vaccine dose: 0.3 mg/kg1
Route of administration: Intravenous
Cargo: siRNA
Buffer: Potassium phosphate, monobasic, anhydrous Sodium phosphate, dibasic, heptahydrate pH ~ 7
N/P ratio: 3
| Lipid Class | Ionizable lipid | PEG | Phospholipid | Sterol |
| Lipid Name | DLin-MC3-DMA | PEG2000-DMG | DSPC | Cholesterol |
| Molar ratio | 50 | 1.5 | 10 | 38.5 |
LNP Composition in the BNT162b2 COVID-19 mRNA vaccine by Pfizer-BioNTech
The Pfizer-BioNTech COVID-19 vaccine, known as BNT162b2, is a groundbreaking mRNA LNP vaccine developed to combat the COVID-19 virus. This vaccine uses messenger RNA technology to instruct cells to produce a protein that triggers an immune response, offering protection against the virus. The intracellular mRNA delivery was successfully achieved using the below LNP composition. It was the most widely used COVID 19 vaccine worldwide.
mRNA vaccine Doses: 30µg1
Route of administration: Intramuscular
Cargo: mRNA
Buffer: 0.01 mg Potassium dihydrogen phosphate 0.07 mg Disodium hydrogen phosphate dihydrate pH 7–8
N/P ratio: 6
| Lipid Class | Ionizable lipid | PEG | Phospholipid | Sterol |
| Lipid Name | ALC-0315 | ALC-0159 (PEG2000) | DSPC | Cholesterol |
| Molar ratio | 46.3 | 1.6 | 9.4 | 42.7 |
LNP Composition in the mRNA-1273 COVID-19 vaccine by Moderna
Likewise, the Moderna COVID-19 vaccine, designated mRNA-1273, is a pioneering development in immunization technology, utilizing messenger RNA (mRNA) combined with LNP to effectively combat the COVID 19 virus. It uses the same working principle of introducing mRNA into the host cell, to replicate the virus’ spike protein, inducing an immune response without the actual virus.
mRNA vaccine doses: 100µg1
Route of administration: Intramuscular
Cargo: mRNA
Buffer: Tris (tromethamine)
pH 7–8
N/P ratio: 6
| Lipid Class | Ionizable lipid | PEG | Phospholipid | Sterol |
| Lipid Name | SM-102 | PEG2000-DMG | DSPC | Cholesterol |
| Molar ratio | 50 | 1.5 | 10 | 38.5 |
How to choose the right lipid nanoparticle composition for your development?
As you have understood so far, there is no universal answer to this question as numerous elements need to be considered to choose your optimal lipid mix for your development including the target organ/cell, the cargo (mRNA, siRNA, saRNA…), the route of administration, the formulation process…
However, here is a set of guidelines to follow when designing your LNP system:
- Oligonucleotide/RNA Type: Choose the RNA type (mRNA, siRNA, sgRNA, ASO) based on your therapeutic goal, as this influences your choice of LNP.
- Lipid Selection: Start with established LNP compositions suitable for different cargoes and targets, then explore more complex options.
- Formulation Design: Select appropriate buffers, pH, and N:P ratio. Typically, lipids in ethanol use an acidic buffer in the aqueous phase for lipid ionization during RNA complexation.
- Mixing Process: Use a microfluidics-based system for effective and homogeneous mixing of aqueous and organic phases, ensuring consistent RNA-LNP complex formation.
- Parameter Optimization: Adjust synthesis conditions like flow rates and concentrations to achieve the right nanoparticle size, PDI, and encapsulation efficiency for effective RNA-LNP intracellular delivery.
- Selectivity Improvement (Optional): Enhance targeting by adding ligands or peptides to LNPs for active targeting to specific cell types.
- Testing and Refinement: Conduct physicochemical characterization, in vivo and in vitro testing, including
Optimize LNP formulation iteratively for each payload and target, refining both composition and manufacturing processes.
Potential improvement for the development of novel LNP
To further optimize your LNP delivery system, you can also explore several more advanced alternative options, either using different types of lipids or by decorating your nanoparticles for active targeting.
Alternatives to PEG
As described previously, PEGylation, the process of attaching polyethylene glycol to nanoparticles, is a common strategy to help stabilize nanoparticles, extend the circulation time of LNP in the bloodstream through the stealth effect and control their size for optimize endocytosis process. However, some concerns about PEG, like potential allergic reactions and the immune system’s growing familiarity with PEG (leading to reduced efficacy), have driven research into alternative materials. Here’s an introduction to some PEG alternatives for LNP formulation, including:
- Polysarcosine (pSar): A peptide-based polymer offering biocompatibility and reduced immune recognition, useful for extending nanoparticle circulation time.
- Hydrophilic Polymers: Such as polyvinyl alcohol and polyvinylpyrrolidone, these provide a ‘stealth’ coating to nanoparticles, similar to PEG.
- Zwitterionic Polymers: Contain both positive and negative charges, reducing non-specific protein binding and enhancing circulation time.
- Polyamino Acid-Based Polymers: Biodegradable polymers like polyglutamic acid, offering reduced immunogenicity for drug delivery.
- XTENylation: A hydrophilic polypeptide alternative to PEG, it can extend the half-life of therapeutic proteins and peptides in the bloodstream.
- Polytioglycidul Glycerol: A newer polymer, adjustable for specific LNP needs, enhancing stability and circulation time. [2]
Using Alternative to cholesterol
Cholesterol is a critical component in liposome formulations, contributing to the rigidity and structural integrity of the lipid bilayer. Recent studies, however, are exploring alternative compounds that can replicate or even enhance the stabilizing effects of cholesterol, with improving delivery efficiency, encapsulation efficiency, and drug release profile.
Most common alternative to PEG currently in use is β-sitosterol, which has an additional alkyl group in comparison to cholesterol [3]
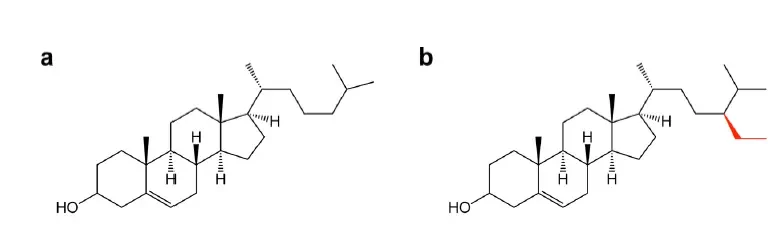
In a recent study by Jeonghwan Kim and all2, they show that β-sitosterol can help improve the expression of luciferase in HeLA cells by 10 to 100 folds compared to regular Cholestrol composition.
Passive and Active Targeting
Active and passive targeting methods can be used to improve the specificity of lipid nanoparticles towards specific tissues or cells, to enhance their therapeutic efficacy, and reduce side effects. Generally, active targeting is achieved by modifying and functionalizing the LNP’s surface with targeting elements, such as ligands, antibodies, or peptides that recognize and bind to specific receptors on the target cells. Instead, passive targeting relies on the optimization of the LNP physicochemical properties (Composition, size…) for a more selective delivery.
Conclusion of Lipid nanoparticle for RNA Therapies
In conclusion, the success of FDA-approved RNA-LNP therapies like Onpattro, Comirnaty, and Moderna’s mRNA-1273 vaccine highlights the vital role of lipid nanoparticles (LNPs) for novel RNA-based vaccine development and therapeutic interventions. The effectiveness of these LNPs stems both from their meticulously engineered lipid compositions, including ionizable lipids for efficient RNA encapsulation, PEGylated lipids for stability and optimal biodistribution, phospholipids for structural integrity, and sterol lipids for membrane flexibility, and their fabrication process, which has a major impact on the final nanoparticle characteristics.
Yet, despite those successes, LNP design for RNA vaccines and therapeutics can further be improved with emerging alternatives to traditional components like PEG and cholesterol, pointing to a future of enhanced efficacy and reduced immunogenicity. Moreover, the incorporation of active targeting mechanisms through surface modifications enhances the specificity of RNA delivery, maximizing therapeutic efficacy while minimizing side effects.
This article highlights the complex nature of LNP design and the cutting-edge scientific progress that propels the field of RNA-based treatments. With the broadening use of LNPs, continuous research, and enhancement of these nanoscale delivery systems are essential to fully realize their capabilities across various medical fields.

Interested in learning more about LNP formulation for RNA therapies development?
Reach out to us to learn how we can help!