Abstract
Lipid nanoparticles (LNPs) are revolutionizing CRISPR-Cas9 delivery by protecting RNA payloads and enhancing cellular uptake.
This guide details key lipid components and highlights microfluidic formulation methods for precise, efficient, and scalable CRISPR therapeutic systems. Emerging biodegradable and tissue-targeted LNPs optimize safety, specificity, and in-vivo gene editing.
Recent advances in biotechnology have revolutionized genome editing methods, particularly through the introduction of CRISPR-Cas9 technology. This system, which enables precise genome modifications, has opened new possibilities in gene therapy, fundamental research, and personalized medicine, revolutionizing medicine such as illustrated by the 2020 Chemistry Nobel Prize. However, one of the major current challenges in the clinical application of CRISPR-Cas9 lies in the efficient and targeted delivery of genome editing components to specific cells.
In this context, Lipid Nanoparticles (LNP) have emerged as a promising solution, playing a key role in optimizing the delivery of CRISPR-Cas9. LNPs, which have already demonstrated their efficacy in mRNA delivery, offer CRISPR-Cas9 components not only protection from nucleic acid degradation but also enable more targeted delivery while minimizing off-target effects. Nanoparticles can be engineered to modify their genetic payload thanks to their precise targeting, scalability, editing efficiency, low immune response potential, and reduced sensitivity to nucleases.
In this review focusing on Lipid nanoparticle for CRISPR-Cas9 delivery, we will first highlight the importance of the discovery of the CRISPR-Cas9 system and its working mechanism. Next, we will examine the composition and design of lipid nanoparticles (LNP). Finally, we will analyze how these LNPs present a particularly interesting method for delivering the CRISPR-Cas9 system.
The CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) Cas9 Revolution
CRISPR-Cas9 has revolutionized biotechnology by making genome editing simpler, faster, and more precise, with major applications in gene therapy, agriculture, and research. It has led to significant advancements in the treatment of genetic and infectious diseases while reducing costs and accelerating scientific discoveries. Since its discovery in 2012, the CRISPR-Cas9 system has become one of the most actively researched fields. In fact, the 2020 Nobel Prize in Chemistry was awarded to Emmanuelle Charpentier and Jennifer Doudna for their work on this technology.
Working principle
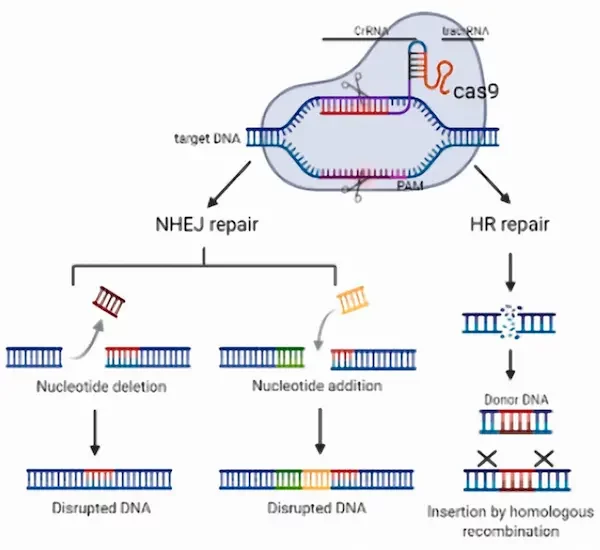
The acronym CRISPR refers to a specific region of the bacterial genome, originally discovered as a defense mechanism against viruses, while Cas9 denotes a protein associated with CRISPR that acts like “molecular scissors,” capable of cutting DNA at precise locations.
The functionality of CRISPR-Cas9 relies on two main components that form the RNP (Ribonucleoprotein) complex :
- Guide RNA (gRNA): A short RNA sequence programmed to target a specific DNA sequence in the genome. This guide RNA binds to the target sequence through base-pair complementarity.
- Cas9 protein: This enzyme is directed by the guide RNA to the target DNA sequence. Once it reaches the designated location, Cas9 cuts the DNA at the precise site identified by the guide RNA.
After the DNA is cut, two types of repairs can occur:
- Non-homologous end joining (NHEJ): This DNA repair mechanism is fast but often prone to errors, which can lead to mutations or gene disruptions that inactivate the target gene.
- Homology-directed repair (HDR): When the cell has a template DNA copy, it can repair the break by inserting a new genetic sequence, allowing for the modification or correction of genetic mutations.
CRISPR Delivery Approaches
As described above, the delivery of Cas9 components into cells is a critical factor for an efficient gene edition. The Cas9 protein can be delivered in three main formats: DNA, mRNA, and protein, each offering unique pros and cons.
- Plasmid DNA is cost-effective and easy to prepare, offering longer expression, which may be beneficial for sustained editing. However, its slower onset and risk of off-target effects and insertional mutagenesis due to prolonged expression are concerns.
- mRNA delivery provides faster gene editing because it bypasses transcription, but it allows only transient Cas9 expression, reducing off-target effects. mRNA’s instability requires chemical modifications to enhance its effectiveness.
- Protein delivery enables immediate action in the nucleus, making it the most efficient for quick gene editing, but its transient presence and higher cost are drawbacks. Additionally, bacterial-origin Cas9 protein raises safety concerns related to immune responses.
Delivery Strategies
Different approaches presented in the table below have been developed to deliver the components of the CRISPR-Cas9 system into cells, each with specific advantages and limitations.
The consideration to take into account for Cas9 delivery are :
- Efficiency: Direct delivery of Cas9 RNP complexes can be efficient but faces challenges in systemic applications due to poor intracellular uptake. Viral vectors can enhance delivery but have limitations in terms of immune responses and packaging capacity.
- Safety: Viral vectors, especially lentiviruses, pose risks related to genomic integration. Adenovirus avoids integration but triggers immune responses. Non-viral methods, such as LNPs and electroporation, offer safer alternatives but may require further optimization for broader tissue targeting.
- Applicability: Not all the methods can be used for in vitro and in vivo gene editing, and offer different levels of invasion. While efficient, approaches such as electroporation being only possible ex vivo offer challenges related to invasion.
The below table recap the advantages and drawbacks of each approaches [1]
| Delivery approach |
Viral Vectors
|
Non viral delivery
|
|||||
|---|---|---|---|---|---|---|---|
| AAV (Adeno-Associated Virus) | Lentivirus | Adenovirus | EV | Microinjection | Lipid Nanoparticles (LNP) | Electroporation | |
| Cas9 Delivery Format | DNA | DNA | DNA | Protein | DNA, mRNA or protein | DNA, mRNA or protein | DNA, mRNA or protein |
| Delivery Efficiency | Low | Medium | Medium | Medium | Low | Medium | High |
| Safety concerns | Medium | High | Medium | Low | Low | Low | Low |
| Advantages | Non integrating. Effective delivery of small DNA sequences. | Efficient delivery. | Non integrating. | All in one format | Direct delivery | Low stress, high encapsulation rates | Efficient delivery |
| Limitations | Limited capacity (~4.7 kb) -> Cannot integrate large sequences. | Risk of unwanted genomic integration. | Strong immune response. Complex | Production difficult, more complex approach | Only ex vivo => Very invasive | Requires optimization | Only ex vivo => Very invasive |
While electroporation has been widely used for ex vivo applications due to its high efficiency, its invasiveness limits practicality for in vivo work. Recent developments in Lipid Nanoparticles (LNPs), especially after their success in mRNA COVID-19 vaccines, are shifting focus towards non-viral methods. LNPs are now considered a leading delivery option for CRISPR/Cas9, with companies like Beam Therapeutics reporting promising results from novel CRISPR/Cas9 formulations.
Overview of LNPs for CRISPR/Cas9 delivery
Lipid nanoparticles (LNPs) have drastically transformed the field of biotechnology and drug delivery, particularly for RNA-based therapies and mRNA vaccines, while also paving the way for other gene therapies involving different types of RNA (such as siRNA and miRNA). By encapsulating RNA, LNPs protect it from enzymatic degradation and enhance its cellular uptake, ensuring RNA stability, efficient cytosolic delivery, and optimal translation into proteins within target cells.
This technology addresses inefficiencies in delivery and immune responses triggered by naked nucleic acids, offering significant advantages over viral vectors, which are often limited by payload capacity and safety concerns. The success of mRNA vaccines for COVID-19 clearly highlights the vast potential of LNPs to improve the delivery and effectiveness of RNA-based treatments, making them essential in modern medicine.
LNP composition
Lipid nanoparticles (LNPs) are primarily composed of four families of lipids:
- Ionizable Cationic Lipids: These play a crucial role in enhancing LNPs for the encapsulation and delivery of nucleic acids. Ionizable lipids acquire a positive charge at acidic pH, allowing them to interact with negatively charged nucleic acids during LNP formation. This property improves encapsulation and protects the mRNA or CRISPR/Cas9 payload from degradation before delivery to target cells. At physiological pH, these lipids are uncharged, which prevents sequestration by immune cells and extends the circulation time of LNPs in the bloodstream. Upon endocytosis, their positive charge re-emerges in the acidified endosomes, facilitating effective cargo release into the cytosol and avoiding degradation by lysosomal enzymes. These lipids enable targeted and efficient delivery of CRISPR/Cas9 components, including mRNA and proteins, while minimizing off-target effects and toxicity.
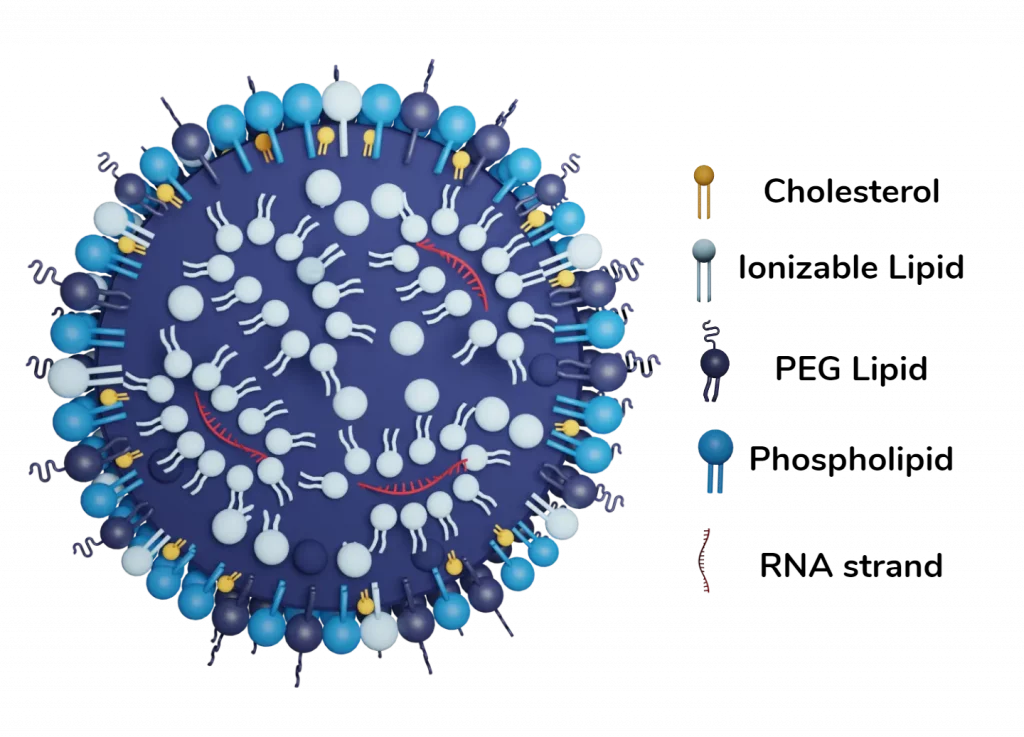
- PEG Lipids: PEG lipids play a key role in enhancing the stability of LNPs by regulating particle size, reducing immunogenicity, and prolonging their circulation time in the body. Their structure, with PEG molecules attached to lipid heads, forms a coating on the outer surface of LNPs that prevents particle aggregation during formulation, storage, and bloodstream circulation. Additionally, it protects LNPs from phagocytosis by immune cells, thereby extending their lifespan in the circulation. These properties help improve the delivery of CRISPR/Cas9 molecules to target cells, increasing gene editing efficiency.
- Phospholipids: These are mainly present in the outer lipid layer of LNPs and are used to enhance structural stability and delivery efficiency. Combinations of phospholipids can be added to LNP formulations to modify their biophysical characteristics, including particle size and surface charge, to promote optimal encapsulation, stability, and endosomal release.
- Cholesterol: Cholesterol is used in LNP formulations to increase particle stability by filling gaps between phospholipids. Cholesterol also promotes membrane fusion during cellular uptake by enhancing the activity of positively charged lipids and facilitating the destabilization of the cell membrane during fusion.
LNP formulation for CRISPR-Cas9 delivery
Several methods exist for formulating LNPs loaded with CRISPR/Cas9 components, with microfluidics emerging as the most efficient and popular approach. The use of microfluidic technologies for LNP formulation allow for precise, low-volume (µL/mL scale) formulations, making them ideal for optimizing CRISPR/Cas9 delivery while conserving costly materials. Microfluidics offers excellent reproducibility, and control over particle characteristics, leading to highly efficient gene-editing formulations with easily optimized and consistent performance. This technology has become essential in drug development due to its ability to streamline the formulation process and improve the therapeutic potential of CRISPR/Cas9 systems.
Additionally, microfluidics methods can be employed to optimize targeting through two main strategies: passive and active targeting.
- Passive targeting focuses on optimizing the physicochemical properties of LNPs, such as size, zeta potential, and other characteristics, to direct them to specific tissues or cells. Microfluidics facilitates the fine-tuning of these nanoparticle properties simply by adjusting formulation conditions.
- Active targeting involves modifying LNPs with ligands, peptides, or other molecules to enhance tissue or organ specificity, and thus avoid liver clearance, thereby improving biodistribution and overall efficacy. This approach enables more precise targeting of therapeutic agents to the desired sites in the body.
LNPs and CRISPR-Cas9: A Promising Duo
The studies compiled in the review *Lipid Nanoparticles: The Game-Changer in CRISPR-Cas9 Genome Editing* by Arezoo Mohammadian Farsani et al. demonstrate that encapsulating and delivering RNPs into target cells through modified LNPs enables efficient genome editing, with promising in vivo applications, such as restoring hearing. However, the large size and sensitivity to denaturation of Cas9 limit the effectiveness of this approach, although improved LNP formulations have been developed to target specific tissues with greater precision.
To enhance the specificity of CRISPR RNA delivery and reduce off-target effects, recent studies have focused on designing biodegradable LNPs with selective cellular targeting. For example, it has been shown that introducing disulfide bonds into the hydrophobic tail of ionizable lipids promotes endosomal escape and intracellular release of Cas9 mRNA.
Recent advancement in CRISPR/Cas9 delivery
Ongoing Research
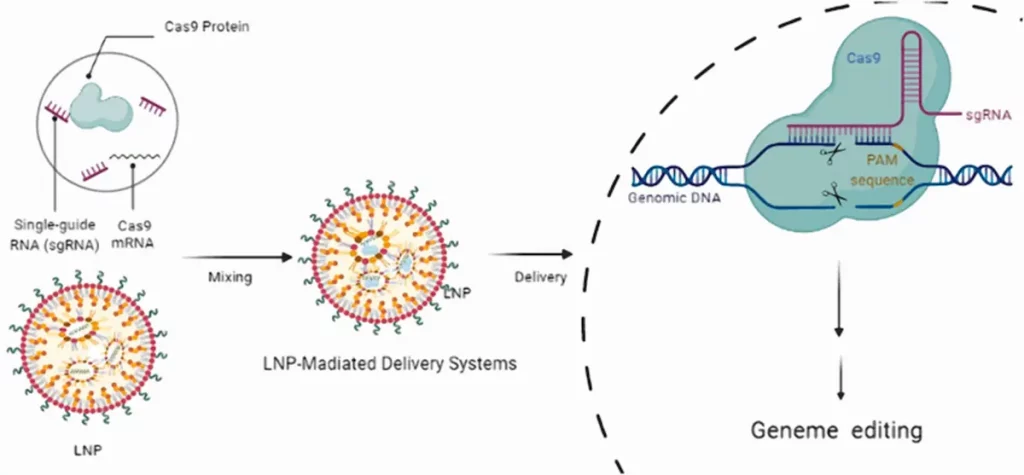
Research is increasingly exploring the delivery of Cas9 mRNA for genetic editing, particularly through lipid nanoparticles (LNPs) that can co-encapsulate Cas9 mRNA and guide RNA (gRNA) in a single particle or encapsulate them separately. Co-encapsulation appears to be more effective, ensuring a better ratio between the two RNAs, which is crucial for genome editing.
For example, Liu et al. used an LNP formulation with the bio-reductive ionizable lipid BAMEA-O16B for rapid and efficient in vitro and in vivo editing, reducing the expression of a targeted protein in cells and in mice with minimal side effects. LNPs have also been used to deliver base editors, such as those targeting the PCSK9 gene, successfully inducing precise genome editing in the liver of mice and macaques with low toxicity.
A study using cationic lipids demonstrated gene editing in human primary hepatocytes, effectively reducing LDL cholesterol levels in primates, confirming the efficacy of LNPs for clinical use.
Other research focuses on gene editing in cancer. For example, Finn et al. developed a formulation, LNP-INT01, for the delivery of Cas9, demonstrating sustained modification of the Ttr gene in mice, with repeated doses allowing precise control of genetic editing. Qiu et al. used LNPs to target the ANGPTL3 gene, reducing cholesterol and triglyceride levels without significant toxicity. Meanwhile, modified LNP systems, such as SORT lipids, enable targeting of specific organs like the lungs and spleen, opening possibilities for selective genetic editing.
Further studies have shown that LNPs targeting cancer cells can be used to modify specific genes, such as HPV18E6, slowing tumor growth. Lastly, LNP systems modified for intramuscular delivery have shown promising results in Duchenne muscular dystrophy models, with effective and repeatable genetic editing.
The use of LNPs for gene therapy is therefore at the forefront of current research. An example is the company Beam Therapeutics, which works with LNPs for the direct in vivo delivery of their base editor. This editor, similar to Cas9, allows for genome correction by rewriting it base by base using a modified CRISPR protein for precise targeting. CRISPR delivers a deaminase to the sequence, which chemically alters the targeted base. This technique is also very promising for new therapeutic programs.
Clinical Trials
The first clinical trial using LNPs for CRISPR/Cas9 was launched in 2020 by Intellia Therapeutics, targeting the TTR gene to treat hereditary transthyretin amyloidosis (ATTRv-PN). The initial results were highly promising, showing a significant reduction in TTR protein levels, marking a major milestone for in vivo CRISPR therapies.
In 2021, Intellia expanded its clinical efforts with a second trial targeting the KLKB1 gene to treat hereditary angioedema (HAE). Preclinical studies in animal models demonstrated encouraging results, leading to high expectations for therapeutic success in humans.
In 2024, Beam Therapeutics began clinical trials for a new base editor, BEAM-302, aimed at treating alpha-1 antitrypsin deficiency (AATD). This new trial highlights the growing potential of LNPs for delivering not just CRISPR/Cas9, but also next-generation genome-editing technologies like base editors. [2]
These efforts underscore the versatility and effectiveness of LNPs as a delivery platform for CRISPR-based gene therapies, with advancements in formulation technology enhancing specificity, safety, and therapeutic outcomes. LNPs have become a key tool in translating genome-editing innovations from preclinical research into clinical practice.
Conclusion on using LNP for CRISPR/Cas9 delivery
Recent advances in biotechnology, particularly with CRISPR-Cas9 technology, have revolutionized genome editing, paving the way for new possibilities in gene therapy, fundamental research, and personalized medicine. However, efficiently and precisely delivering CRISPR-Cas9 components to specific cells remains a significant challenge. In this context, lipid nanoparticles (LNPs) have emerged as a promising solution, providing protection against nucleic acid degradation and enabling targeted delivery, thereby minimizing off-target effects.
LNPs, which have already been successfully used for mRNA delivery, enhance the effectiveness of the CRISPR-Cas9 system by encapsulating nucleic acids, extending their circulation time in the body, and optimizing their intracellular release. Their precise design, low immune response potential, and efficient targeting make them an increasingly favored delivery method for clinical applications of CRISPR. Overall, integrating LNPs into genome-editing approaches opens up vast opportunities, particularly for targeted therapies and promising clinical trials. Continuous optimization of this technology could revolutionize treatments for various genetic disorders and contribute to the rise of precision medicine.
If you’d like to explore a practical approach to using LNPs for CRISPR applications, take a look at our application note: “Benchmarking LNP vs Electroporation for eGFP RNA and CRISPR-Cas9 Delivery in HSCs”

Interested in using LNP for CRISPR-Cas9 delivery?
Reach out to us to learn how we can help!
References
[1] Yip BH. Recent Advances in CRISPR/Cas9 Delivery Strategies. Biomolecules. 2020 May 30;10(6):839. doi: 10.3390/biom10060839. PMID: 32486234; PMCID: PMC7356196.
[2] https://investors.beamtx.com/news-releases/news-release-details/beam-therapeutics-presents-lnp-formulation-data-asgct-and/