Abstract
Endosomal escape is a pivotal barrier in LNP-mediated therapeutics, determining whether RNA payloads reach the cytoplasm for successful translation. This review explains how lipid composition—especially ionizable lipids—facilitates escape through membrane destabilization and phase transitions. It also evaluates strategies to enhance endosomal escape of LNP and outlines imaging and reporter-based techniques to detect and quantify this essential step
Endosomal escape of lipid nanoparticles (LNPs) is a crucial process in the intracellular delivery of mRNA-based therapies and vaccines. After cellular uptake via endocytosis, LNPs must efficiently release their mRNA payload from endosomes into the cytoplasm to ensure proper translation into therapeutic proteins. This step is vital for the success of LNP-mediated delivery systems, as it overcomes the barriers posed by endosomal entrapment and degradation, thereby enhancing the overall efficacy and potency of mRNA therapeutics.
In this article, we will start with a reminder on LNPs for nucleic acids delivery. We will then explore the endo-lysosomal pathway that LNPs undergo once inside cells. Following this, we will discuss two theories of endosomal escape mechanisms influenced by the composition of LNPs. After that, we will examine various approaches to achieve an enhanced endosomal escape and conclude with an overview of detection methods used to monitor endosomal escape.
Reminder: Lipid Nanoparticles for Nucleic Acids Delivery
LNPs have revolutionized the biotechnology and drug delivery field, particularly for RNA-based therapies and mRNA vaccines, and opened the doors to numerous other gene therapies using other RNA types (siRNA, miRNA…). By encapsulating RNA, LNPs protect against enzymatic degradation and enhance cellular uptake, ensuring RNA stability, effective cytosolic delivery, and translation into proteins within target cells. This technology addresses delivery inefficiencies and immune responses associated with naked nucleic acids and gene delivery, offering advantages over viral vectors limited by payload capacity and safety concerns. The success of COVID-19 mRNA vaccines highlights the potential of LNPs to improve the delivery and efficacy of RNA-based treatments and RNA therapeutics making them crucial in modern medicine.
The composition of LNPs, particularly the inclusion of ionizable lipids, plays a major role in regulating endosomal escape. Those ionizable lipids have the unique capability of switching charge with the pH and being positively charged at low pH while remaining neutral at physiological pH. Their use allows for improved delivery: upon entering the endosome, where the pH value drops, lipids get positively charged, leading to a destabilization of the particle and thus facilitating easier delivery of the nucleic acid into the cytoplasm.
Understanding the mechanism of endosomal escape and optimizing ionizable lipids are essential for the efficacy and success of RNA-based therapies.
Unlocking the Basics: An introduction to the Endo-Lysosomal system
The Endo-Lysosomal system plays an essential role in the intracellular fate of LNPs, influencing their internalization, trafficking, and eventual release of cargo within the cell.
The 4 steps of the LNP internalization process:
- Endocytosis
LNPs enter cells through various endocytosis pathways, including both clathrin-dependent and clathrin-independent mechanisms like macropinocytosis, depending on the LNP size. Macropinocytosis generally predominates as the primary route for LNP internalization. Once internalized, LNPs follow the endocytic pathway, being transported to early endosomes, which then progress to late endosomes and fuse with lysosomes [1]. - Early Endosomes
Early endosomes represent the initial compartment where the internalized cargo resides following cell endocytosis. These endocytic vesicles are characterized by a slightly acidic pH of approximately 6.2, regulated by a V-ATPase proton pump and the presence of various RAB proteins in their GTP-bound state (RAB 4, 5, 10, 14, 21, 22, EEA1), which facilitate endosomal fusion and tethering. While most of the cargo internalized by early endosomes undergoes recycling, only a portion is directed towards late endosomes and subsequently to lysosomes [1]. - Late Endosomes
The transition from early endosomes to late endosomes involves a series of sequential events. These include the activation of RAB proteins (from RAB5 to RAB7), acidification of the endosomal lumen (from pH~6.2 to pH 6-4.9) and acquisition of lysosomal hydrolases and membrane proteins [1]. - Lysosomes
Late endosomes can then fuse with lysosomes, forming endolysosomes, where degradation occurs. The acidic environment within endolysosomes activates hydrolytic enzymes, facilitating the breakdown of molecules [1].
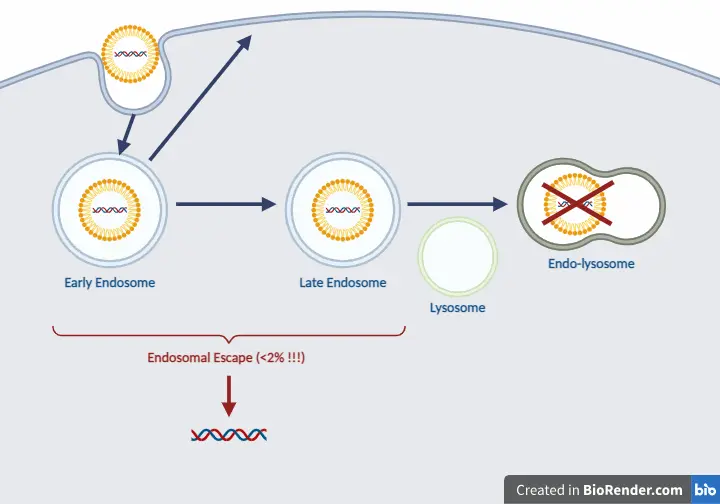
Having outlined the endo-lysosomal system that LNPs navigate once inside cells, we can now delve into the critical phase of endosomal escape. This step is pivotal for the effective release of therapeutic payloads into the cytoplasm. Understanding the mechanisms behind endosomal escape is essential for optimizing LNP efficacy and enhancing therapeutic outcomes.
Mechanisms of Endosomal Escape
There are two main theories that explain the mechanisms of endosomal escape for LNPs, both influenced by their lipid composition. These theories highlight how specific lipid components can facilitate the release of the therapeutic payload into the cytoplasm, to optimize the intracellular delivery.
Membrane Phase Transition
Ionizable lipids make up 50-70% of the components in LNPs, significantly determining their physical and chemical characteristics, particularly their role in facilitating endosomal escape.
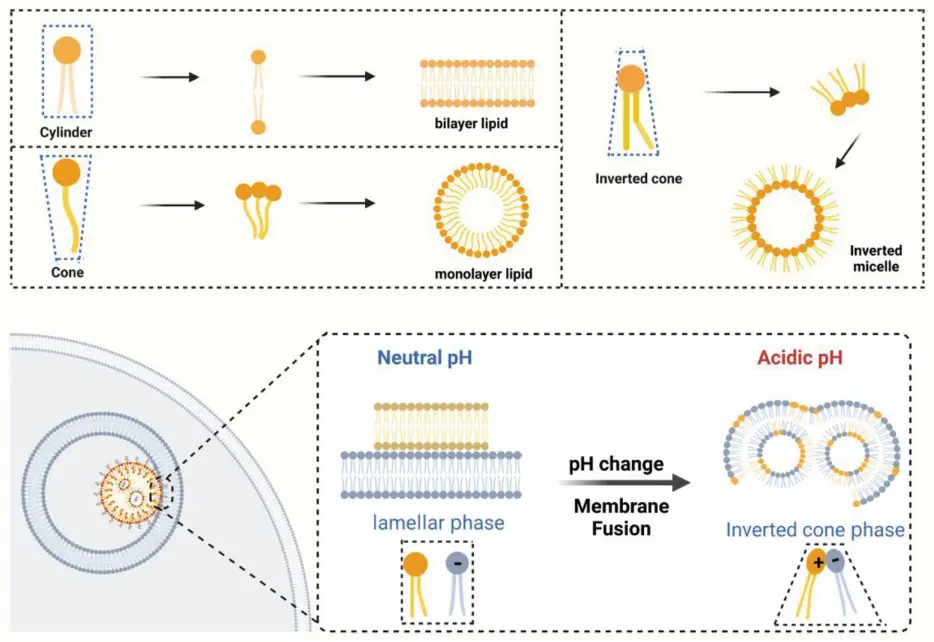
The ratio of the length to width of the hydrophobic tail to the head determines the assembly mode of the lipid molecule. There are 3 different assembly modes [2]:
- When the size of the hydrophilic head of lipid molecules matches the width of the hydrophobic tail, the lipid molecules arrange themselves in a bilayer structure when dispersed in water
- If the head exceeds the width of the hydrophobic tail, the lipid molecule will disperse in water as micelles, with the hydrophilic portion oriented outward on the surface of the micelle
- When the hydrophilic head of lipid molecules is smaller than the width of the hydrophobic tail, the lipid molecules still disperse in water as micelles. In this case, the hydrophilic cores of the molecules are oriented inward, forming the core of the micelle structure
When LNPs are internalized by cells through endocytosis, they are encapsulated in endosomes. The internal environment of the endosome becomes increasingly acidic due to proton pumps. Ionizable lipids in the LNPs, which are designed to remain neutral at physiological pH, become protonated in this acidic environment, altering their charge and conformation. The positively charged heads of ionizable lipids interact with the negatively charged lipids present on the luminal side of the endosomal membrane. This interaction induces a transition from the lamellar phase to the inverted cone phase, leading to the fusion of endosomal membranes with LNP lipid components. The fusion and subsequent destabilization of the membranes allow the release of the encapsulated therapeutic agents into the cytoplasm, facilitating effective delivery and avoiding lysosomal degradation [1,2].
To sum up, the critical function of LNP endosomal escape is ionizable lipids. These components disrupt the integrity of the initial endosomal membrane, leading to the formation of a non-lamellar hexagonal structure. Consequently, this induces the fusion of the membranes and rupture of the endosome, enabling the nucleic acid payloads to be released in the cytosol. Thus, LNPs must operate in an acidic environment to effectively deliver nucleic acids.
Proton Sponge Effect
The proton sponge effect is a mechanism by which cationic polymer nanoparticles, like polyethylenimine (PEI), facilitate endosomal escape. When these nanoparticles are internalized by cells via endocytosis, they become trapped in endosomes, which gradually acidify due to the action of proton pumps that lower the internal pH. The secondary and tertiary amine groups in cationic polymers become protonated in this acidic environment, leading to a high buffering capacity. This protonation induces the continuous influx of more protons resulting in increased membrane potential. To maintain membrane equilibrium, chloride ions diffuse into the endosomal compartment, further elevating the osmotic pressure within the endosome. As water rushes in to balance this osmotic gradient, the endosome swells and eventually ruptures. This rupture releases the nanoparticles and their nucleic acid payloads into the cytosol, allowing them to avoid lysosomal degradation and exert their intended biological effects [1,2].
Understanding the mechanisms of endosomal escape is crucial for enhancing LNP delivery systems. Researchers have developed various strategies to improve this process, and hence maximize transfection efficiency. The next section will delve into these innovative approaches and their potential to optimize LNP-based therapies.
Approaches to Enhance the Efficiency of LNPs Endosomal Escape
pH-responsive ionizable lipids have largely supplanted cationic lipids owing to their reduced toxicity and enhanced efficacy. These ionizable lipid molecules interact with the endosomal membrane, facilitating its destabilization and turnover. To enhance endosomal escape, 3 approaches are pursued: designing of new ionizable lipids, optimizing other lipid molecules, and incorporating auxiliary materials.
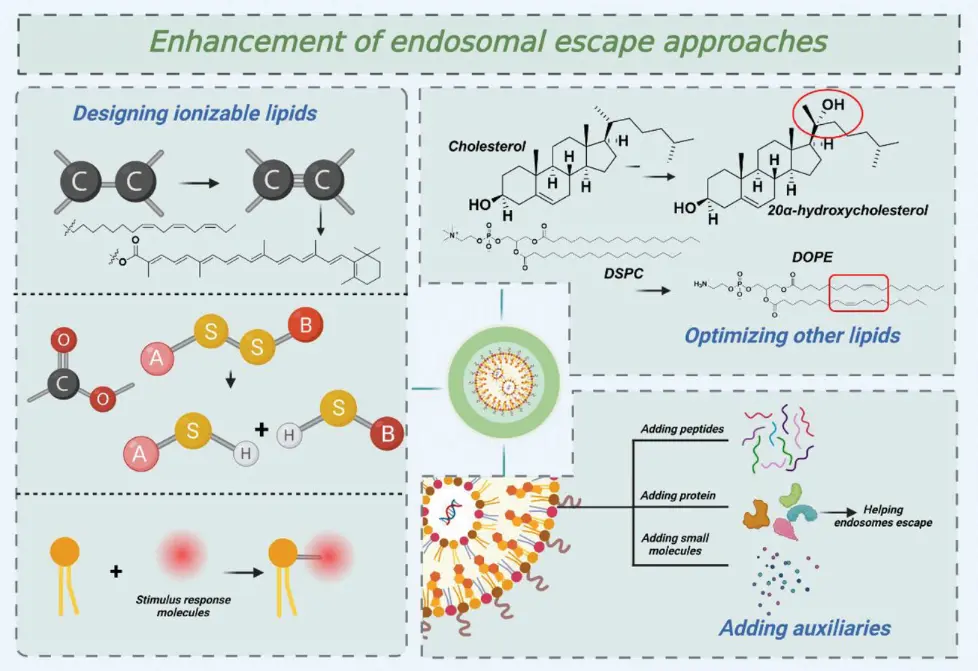
Designing Ionizable Lipids
- Adding Unsaturation:
According to the theory of membrane phase transition, the efficiency of the formation of a non-lamellar hexagonal structure is significantly influenced by the size of the hydrophobic tail. When an unsaturated bond is introduced into the hydrophobic tail, the steric hindrance of the cis-double bond increases the width of the hydrophobic part, which leads to the hexagonal structure and facilitates endosomal escape [2]. - Adding Degradable Groups:
For the delivery of nucleic acids, LNPs need to be biodegradable. The addition of degradable groups in the lipid molecules composing LNPs improves endosomal escape by enabling a decomposition of the lipid molecules, leading to the disintegration of the whole nanoparticle and a release of nucleic acids. Among lipid molecules, ester and disulfide bonds are commonly utilized as degradation units [2]. - Adding Bioactive Molecules:
Biomolecules are mostly derived from organisms, so they have certain biocompatibility and are less likely to cause biological rejection. Novel ionizable lipids derived from natural aminoglycosides called « Aminoglycoside-derived cationic lipids » are biomolecules. They offer biocompatibility and reduce cytotoxic effects, making them safer for clinical applications. The presence of ester bonds in their linkage portions enhances endosomal escape of nucleic acids. When the LNPs containing these ester-functionalized lipids are internalized into endosomes, the acidic pH or enzymatic activity within the endosome facilitates the hydrolysis of the ester bonds. The hydrolysis of the ester bonds leads to the degradation of the lipid molecules into smaller fragments. This breakdown disrupts the lipid bilayer structure of both the LNPs and the endosomal membrane, promoting lipid mixing and membrane fusion. The degradation products can intercalate into the endosomal membrane, destabilizing it further. This triggers the more efficient endosomal escape of nucleic acids [2].
Optimizing Other Lipids
Cholesterol and phospholipids, essential for assembling LNPs, can be optimized to enhance the escape efficiency from endosomes.
- Cholesterol analogs :
Sterol lipids like cholesterol serve as helper lipids, enhancing membrane stability when combined with phospholipids. This stability reduces drug leakage and improves drug stability over time. The length of the alkyl tail, the flexibility of the sterol ring, and the polarity caused by the -OH group can be optimized. Β-sitosterol, a cholesterol analog with alkyl substitution at the C-24 position, promotes endosomal escape. Its structure can disrupt the lipid packing of the endosomal membrane, making it more permeable and facilitating the escape of the LNP contents into the cytoplasm [2]. - Phospholipids
Phospholipids were the predominant components of the original lipid-based nanoparticles from which LNPs derive, constituting nearly 85 mol%. Since then, their role and mol% have decreased in favor of ionizable lipids. Current commercial LNP formulations contain around 10% phospholipids, typically in the form of DSPC. Mostly found in the membrane, phospholipids primarily contribute to enhancing encapsulation efficiency.
Compared to DSPC-LNPs, DOPE-LNPs achieve more efficient endosomal escape due to the structural and functional properties of their constituent lipids.
DOPE contains unsaturated acyl chains and a phosphoethanolamine (PE) headgroup, promoting membrane fluidity and the formation of non-lamellar structures like the inverted hexagonal phase. These properties induce membrane curvature and tension, facilitating lipid mixing and membrane fusion with the endosomal membrane, thereby enhancing the release of encapsulated nucleic acids into the cytoplasm. In contrast, DSPC, with its saturated acyl chains and phosphocholine (PC) headgroup, forms rigid, stable bilayers that resist destabilization and fusion, leading to poor endosomal escape efficiency [2].

Get access to the state of the art microfluidic for RNA-LNP formulation
Incorporating Auxiliaries
Adding auxiliary molecules such as proteins and peptides to lipid nanoparticles can significantly enhance endosomal escape.
- Niemann-Pick disease type C1 (NCP1) Protein :
By incorporating NPC1 into LNPs, NPC1 can affect the maturation process of endosomes, potentially delaying the fusion of endosomes with lysosomes and providing a larger window for the LNPs to escape before degradation [2]. - Cell-penetrating Peptides (CPPs) :
CPPs can directly translocate across the cell membrane, carrying the LNPs into the cytoplasm. This ability to cross cell membranes reduces the dependency on endocytosis and helps bypass endosomal entrapment. These proteins interact with the lipid bilayer of the cellular membrane due to their amphipathic and cationic nature, leading to temporary destabilization and translocation into the cytoplasm [2]. - Protamine :
Protamine, a polycationic peptide, is known for its strong interaction with nucleic acids and its ability to condense them into compact structures. When incorporated into lipid nanoparticles (LNPs), protamine can form stable complexes with nucleic acids. These stable complexes are more efficiently taken up by cells and protected from enzymatic degradation within the endosome, increasing the likelihood of successful endosomal escape and delivery of the nucleic acid payload [2]. - PolyHistidine :
Adding poly-histidine to lipid nanoparticles enhances endosomal escape through the proton sponge effect, driven by the polymerization of histidine due to its nitrogen atoms. These polymers, rich in positively charged nitrogen, act as buffering agents in the acidic endosome, leading to osmotic swelling and membrane disruption. This facilitates the release of LNP contents into the cytoplasm, improving therapeutic payload delivery [2].
After exploring strategies to enhance LNP endosomal escape, it is crucial to assess their effectiveness. The next section will discuss methods used to detect and measure endosomal escape.
Methods to Detect Endosomal Escape
At present, most techniques to detect endosomal escape rely on imaging to either directly observe encapsulated payloads or indirectly identify surrogate markers indicating escape events.
Direct Imaging
For direct imaging of encapsulated payloads, advancements in microscopy allow for the study of internal RNA-LNP trafficking at higher resolutions. This is typically achieved using fluorescently labeled payloads or gold RNA-tagged LNPs, which are imaged using transmission electron microscopy.
- pH-sensitive dye-based microscopic studies: The distinct pH characteristics of endosomal compartments can be utilized for their detection by tagging small molecules with pH-sensitive probes.
Endosome profiling experiments using these probes can uncover variations in endosome properties and functions, such as morphology, localization, uptake, trafficking, endolysosomal pH, and recycling. Furthermore, studying the colocalization of a pH-sensitive probe with fluorescently labeled mRNA-LNPs can identify defects in endosomal organization and the trafficking speed of LNPs from the endosome to the lysosome.
These studies highlight the significance of using pH-sensitive dye-based screening strategies before developing nucleic acid delivery applications. This approach helps ensure a better therapeutic benefit of the payload in the targeted cells [1]. - Endocytic marker-based microscopic studies:
The introduction of advanced confocal and electron microscopes, along with various fluorescent probes to label different compartments of the endomembrane system, has significantly improved our understanding of the intricate processes involved in endosomal escape.
Electron microscopy imaging of gold RNA-tagged LNPs is conducted to determine the compartment of endosomal escape and its characteristics. These characteristics can aid in predicting how LNPs interact with membranes and can be employed to design lipids that achieve higher escape efficiency.
TIRF (Total Internal Reflection Fluorescence) microscopy and smFISH (Single Molecule FISH Imaging) techniques are utilized to detect a single molecule of fluorescently labeled mRNA.
Additionally, SMLM (Single-Molecule Localization Microscopy) is employed to observe single LNPs and their subendosomal localization.
Moreover, FRET (Fluorescence Resonance Energy Transfer) is used to measure the kinetics of LNP disassembly.
Furthermore, microscopic studies using endocytic markers enable the investigation of whether there is a correlation between LNP efficiency and the inhibition of endosomal acidification/maturation, and whether this method can yield promising results for lipid screening assays [1].
Indirect Imaging
The indirect method often involves imaging endosome damage indicators to detect endosomal escape.
- Membrane-based studies
Studying the interactions between LNPs and endosomal membranes is challenging due to the small size of the organelle and LNPs, the dynamic nature of these vesicles, and the complex structure of the membrane. To tackle these challenges, membranes are constructed to mimic the characteristics of endosomal membranes, facilitating the study of their biophysical interactions. In situ optical reflectometry techniques, like ellipsometry, are then employed to gain insights into the degree of interaction and lateral variances by analyzing changes in the polarization of reflected light at the interface. Complementarily, Brewster angle microscopy provides valuable information on the lateral morphology of the monolayer.
However, it is important to note that these membranes are composed solely of lipids, lacking proteins and other components present in endosomes. Furthermore, these models represent flat membranes without the curvature typical of circular endosomes. Last, factors such as the presence of nearby molecules and the influence of various genes affecting escape may impact lipid interactions, aspects not fully captured by these models [1]. - Endosomal damage as a reporter of escape processes:
In the context of endosomal escape, Galectin-based reporter systems are engineered to utilize Galectin proteins (such as Gal8 or Gal9) that bind to glycans present on the inner leaflet of endosomal membranes. These glycans are typically inaccessible until endosomal rupture occurs. Upon endosomal rupture, the Galectin proteins interact with these exposed glycans, activating a reporter signal, such as green fluorescent protein (GFP) or luciferase.
By monitoring the activation of the reporter signal, researchers can track the dynamics of endosomal escape in real-time [1]. - Genetic manipulation of endosomal maturation facilitating proteins:
Another strategy involves genetically manipulating the expression of proteins that promote endosomal maturation at specific stages, aiming to reduce their levels. Scientists have conducted sequence-specific CRISPR knock-out studies to determine the endosomal state of escape. For instance, they have targeted the expression of RAB proteins involved in the endo-lysosomal system at particular stages to observe the resulting effects on endosomal escape [1].
Conclusion on Endosomal Escape
Understanding and better controlling endosomal escape remains a critical challenge in the field of LNP-mediated therapeutics to maximize RNA delivery and transfection efficiency. Understanding the endo-lysosomal system is the first step in addressing this challenge, providing insights into the intracellular pathways that LNPs navigate. By examining the mechanisms of endosomal escape, we can identify how lipid composition influences the release of therapeutic payloads. Building on this knowledge, various approaches have been developed to enhance the efficiency of endosomal escape, optimizing the performance of LNPs in delivering mRNA-based therapies. Finally, accurate methods to detect endosomal escape are essential for evaluating these strategies and guiding further advancements. Together, these elements form a comprehensive framework for overcoming the barriers of endosomal escape, ultimately improving the efficacy of LNP mediated therapeutics.

Looking for the right LNP formulation solution?
Reach out to us to learn how we can help!
References
[1] S. Chatterjee, E. Kon, P. Sharma, et D. Peer, « Endosomal escape: A bottleneck for LNP-mediated therapeutics », Proc. Natl. Acad. Sci. U.S.A., vol. 121, no 11, p. e2307800120, mars 2024, doi: 10.1073/pnas.2307800120.
[2] Y. Jia, X. Wang, L. Li, F. Li, J. Zhang, et X. Liang, « Lipid Nanoparticles Optimized for Targeting and Release of Nucleic Acid », Advanced Materials, vol. 36, no 4, p. 2305300, janv. 2024, doi: 10.1002/adma.202305300.