Abstract
Poly(lactic-co-glycolic acid) i.e. PLGA nanoparticles (PLGA NPs) are biodegradable, FDA/EMA-approved drug delivery systems with tunable size, release kinetics, and targeting capabilities. Versatile in encapsulating hydrophilic and hydrophobic drugs, they are produced via methods like emulsion, spray drying, and microfluidics, the latter offering precise control for nanomedicine applications.
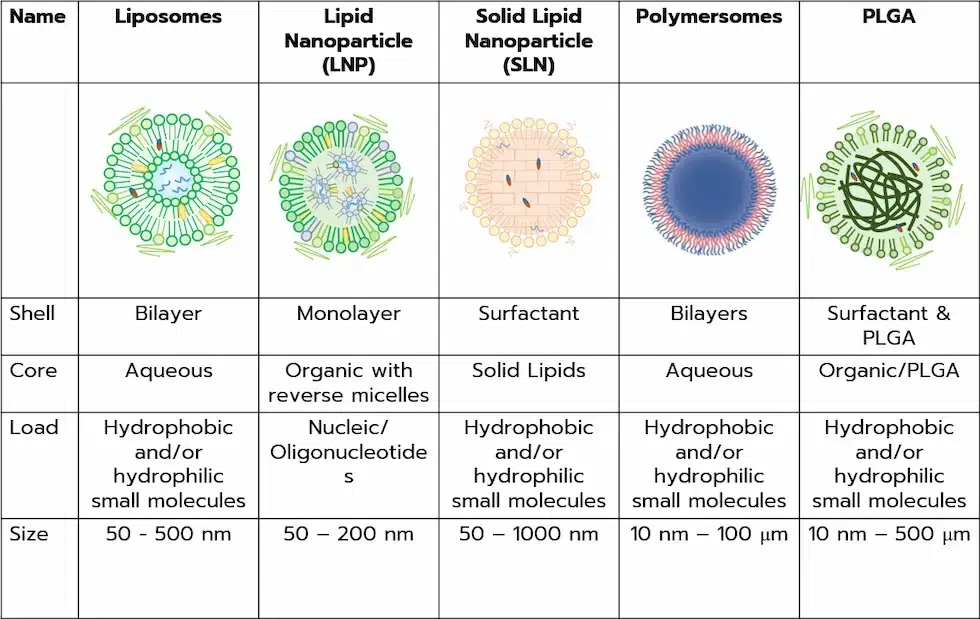
For the past decades, modern medicine shifted more and more towards the use of nanocarriers as drug delivery systems (DDS) to overcome the inherent drawbacks of traditional drug administration. To that end, several drug carriers were developed, ranging from lipid nanoparticles (LNPs), and solid nanoparticles (SLNs), to polymersomes, liposomes, and polymeric nanoparticles. On the latter, a considerable amount of research has been focused on the use of biodegradable polymers to improve the biocompatibility, cytotoxicity, and versatility of nanoparticles DDS.
Among the best candidates, poly(lactic-co-glycolic) (PLGA) has been extensively studied and characterized asa as it is one of the few commercially available biodegradable polymers that is both FDA and EMA-approved. Furthermore, it can easily be tailored to encapsulate several types of drugs (hydrophilic or hydrophobic), and tuned to optimize release and pharmacokinetics. In addition, both PLGA nanoparticles and microparticles can easily be obtained using a wide variety of methods, such as emulsion, spray drying, and electrospray of microfluidic nanoprecipitation.
Using PLGA nanoparticle as a Drug Delivery System
PLGA is a copolymer obtained by the polymerization of lactic acid (LA) and glycolic acid (GA), and can be hydrolyzed and then metabolized into carbon dioxide and water in vivo. This well-reported biodegradability and safety ensures that PLGA formulations are recognized by the European Medicines Agency (EMA) and the American Food and Drug Administration (FDA).
The LA/GA ratio is crucial in tuning the final properties of PLGA NPs as drug delivery systems : the more LA is used in the PLGA formulation, the more hydrophobic the particle will be, and the more GA is used, the more hydrophilic the polymer will be. This ratio will also impact several aspects of the particle, such as its degradation rate (controlled pharmacokinetics) or its encapsulation efficiency regarding either lipophilic or hydrophilic cargos. For instance, a 50:50 PLGA tends to degrade faster than other ratios, resulting in a faster release of the NPs’ payload. Studies also show that the molecular weight and the end modifications of PLGA will impact the properties of the particles, being their degradation rate or the encapsulation efficiency towards different types of molecules (small molecules, peptides, nucleotides, …). However, PLGA nanoparticles are not well suited for the delivery of nucleic acids, and another drug carrier, the LNP, should be used for them.
With that in mind, several drug delivery systems have been developed using PLGA as a carrier, and some of them are reaching preclinical, clinical trials, or even commercialization. In the field of neurodegenerative diseases for instance, some pharmaceutical formulations are being developed to deliver specific payloads (cholesterol, rapamacyn, curcumin,…) to tackle issues like Parkinson’s disease, Alzheimer’s disease, or multiple sclerosis [1].
Cancer treatment, such as prostate cancer and breast cancer are other topics gaining momentum thanks to the numerous clinical trials or even FDA-approved PLGA-based formulations [2]: Eligard, Decapeptyl, Lupron Depot, Zoldaex, …
Cancer research as a whole is benefiting from the use of PLGA np as a nanocarrier, with many more studies focusing on treatments for brain tumors, colon cancer, or cervical cancer, just to name a few [3]
Finally, PLGA-based nanoparticles are also a promising tool for novel therapeutic approaches to major public health concerns, such as antibiotic resistance or respiratory diseases. [4]
PLGA nanoparticles characteristics
As mentioned previously, PLGA nanoparticles can be tailored and used as a nanomedicine platform to address several issues. To that end, their structure can be modified to best suit the applications: size, zeta potential, drug loading, active targeting, probe, therapeutic agent, stealth. For instance, the nanoparticles can be modified with PEG moieties to promote their stealth properties. This PEGylation process promotes immune system evasion, allowing a longer circulation time and thus high probability of binding to the cell membrane before entering cells [5]. The PLGA nanoparticles’ surface can also be functionalized using active targeting methods using ligands or peptides for instance to improve drug release in specific locations in the body.
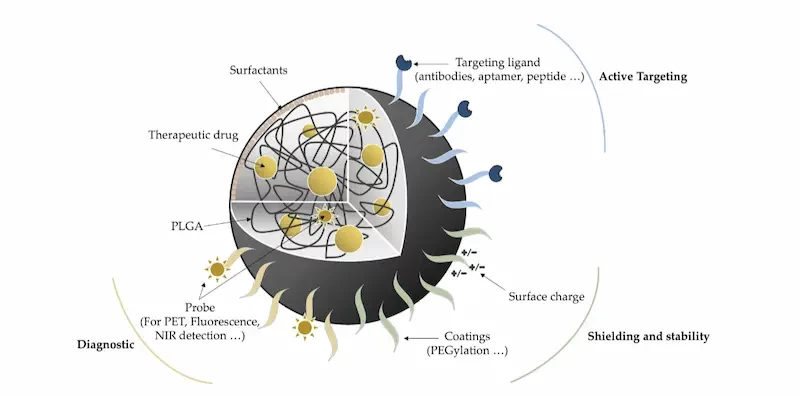
This versatility is so vast that PLGA can be used together with other components to create hybrid PLGA nanoparticles, such as lipid-PLGA nanoparticles, PLGA-oil hybrid nanoparticles, and lipid-PLGA-polymer hybrids … [6]
PLGA nanoparticles Manufacturing methods
Traditionally, PLGA nanoparticles are synthesized using a wide array of methods like emulsification, coacervation, precipitation, spray drying, electrospray or microfluidics. More details can be found in our nanoparticle manufacturing method review.
Emulsion
The most commonly used methods of preparation for PLGA NPs are single and double emulsions, which are suitable for the encapsulation of different types of drugs. The single emulsion method (oil in water, O/W) is more adapted to hydrophobic drugs while the double emulsion technique (water in oil in water, W/O/W) caters more to hydrophilic molecules. Briefly, PLGA is dissolved into an organic medium containing the drugs and then emulsified with an aqueous phase containing surfactants/stabilizers, before evaporation of the organic solvent to obtain the nanoparticles. In the double emulsion technique, the hydrophilic drug is first dissolved into the aqueous phase, emulsified with the organic phase containing PLGA, and then finally mixed with the second aqueous phase containing surfactants. The main drawbacks of the emulsification technique as a whole is poor drug encapsulation efficiency and poor size distribution.
Coacervation
The coacervation method is a liquid phase separation technique involving the use of a hydrophilic medium to reduce the solubility of the materials an cause them to agglomerate into particles (monocoarcevation).
Spray-drying
This process is a fast and convenient way to obtain PLGA NPs, which can also be easily scaled up. It requires the formation of an water-in-oil or solid-in-oil emulsion, which is then going through an atomiser and is dried in a hot heated medium to get the particles. This method can be used for hydrophilic as well as hydrophobic drugs, but can lead to the loss of compounds on the walls of the chamber.
Electrospray
The technique involves a capillary nozzle that is loaded with the polymer solution. Voltage is applied to the nozzle, and the solution is ejected, forming droplets. The size of the particles can be tailored and this technique allows the encapsulation of sensitive compounds.
Nanoprecipitation
The nanoprecipitation technique might be the simplest method to implement in the laboratory as the polymer solution containing the drugs is simply added dropwise to the aqueous medium thus forming nanoparticles. It is a low-cost way to rapidly obtain drug-loaded PLGA particles albeit limited as it is not suitable for hydrophilic active compounds.
Microfluidics
Microfluidics is defined as the manipulation of liquids at very small scales. At these scales, liquids are manipulated in microchannels under laminar flow conditions, which allows for excellent control of the conditions of experiment, such as mixing, heat, and flow.
In the context of nanoprecipitation, the use of microfluidics allows for the highest level of control of the synthesis interaction conditions, therefore increasing the control of the final nanoparticle characteristics. This property has made nanoprecipitation by microfluidics the state-of-the-art method to synthesize well defined nanoparticles. Read more on microfluidic formulation of nanoparticles in our review.
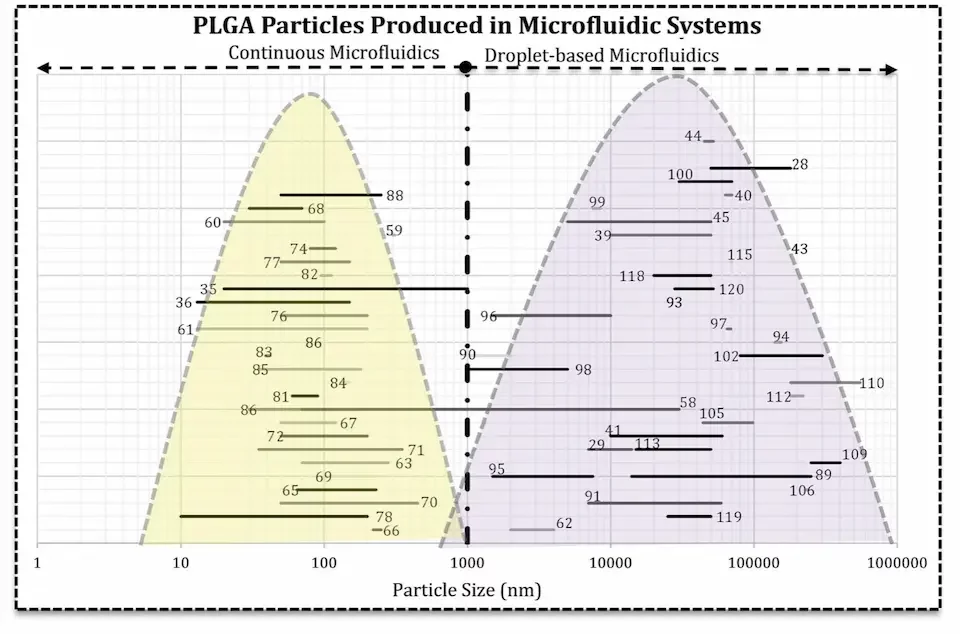
Regarding drug-loaded PLGA particles, microfluidics has been employed in a wide array of applications, reaching promising results. Multiple methods using microfluidics are available, and can broadly be divided into two categories depending on the sought particle size : continuous flow or droplet-based microfluidic.
Continuous flow microfluidics for PLGA nanoparticles:
The continuous flow method, on the other hand, allows for PLGA nanoparticle formulation. In these methods, 2 or more liquids are mixed at microscale to allow for both excellent control and repeatability of the mixing conditions, and reduced mixing time These parameters are extremely important as they lead to controlled homogeneous conditions to obtain nanoparticles, and thus to smaller size distributions.
Droplet microfluidics for PLGA microparticles:
When aiming at micrometer-size PLGA particles, the droplet-based microfluidics methods, droplets of the drug-containing polymer solution are formed in the surfactant-containing aqueous phase thanks to the instability of the inner flow. Tuning important parameters, such as the geometry of the channels, the viscosity of the fluids used, or the flow rate, will enable the user to tune the formation of these PLGA microspheres and thus their characteristics.
PLGA-based DDSs have been beneficiating from microfluidics improvements for the past few decades, and several drugs have been loaded in those particles, such as bupivacaine, ibuprofen, or doxorubicin (DOX) to name but a few.
Conclusion on PLGA nanoparticles formulation
PLGA nanoparticles are a promising delivery system for several types of drugs as well as promising new therapeutical approaches, such as oligonucleotides or peptide derivatives. Indeed, PLGA NPs have not only been proved to be biodegradable, but are also FDA and EMA approved – making them good candidates as nanomedicine platforms.
Their physicochemical parameters (size, PDI, EE%…) can be tuned to best suit their applications, and can be decorated to promote stealth or active targeting. The choice of PLGA NPs manufacturing methods should therefore take those requirements into account. For this reason, manufacturing methods providing good control over these critical parameters should be preferred, and microfluidic’s unique ability to finely control final characteristics, makes it one the state-of-the-art manufacturing methods.

Looking to improve your PLGA formulation process?
Reach out to us to learn how we can help!
References
[1] Cunha, A.; Gaubert, A.; Latxague, L.; Dehay, B. PLGA-Based Nanoparticles for Neuroprotective Drug Delivery in Neurodegenerative Diseases. Pharmaceutics 2021, 13, 1042. https://doi.org/10.3390/pharmaceutics13071042
[2] Lim, Y.W.; Tan, W.S.; Ho, K.L.; Mariatulqabtiah, A.R.; Abu Kasim, N.H.; Abd. Rahman, N.; Wong, T.W.; Chee, C.F. Challenges and Complications of Poly(lactic-co-glycolic acid)-Based Long-Acting Drug Product Development. Pharmaceutics 2022, 14, 614. https://doi.org/10.3390/pharmaceutics14030614
[3] Su, Y. et al. (2021) ‘PLGA-based biodegradable microspheres in drug delivery: recent advances in research and application’, Drug Delivery, 28(1), pp. 1397–1418. doi: 10.1080/10717544.2021.1938756.
[4] Guo X, Zuo X, Zhou Z, Gu Y, Zheng H, Wang X, Wang G, Xu C, Wang F. PLGA-Based Micro/Nanoparticles: An Overview of Their Applications in Respiratory Diseases. International Journal of Molecular Sciences. 2023; 24(5):4333. https://doi.org/10.3390/ijms24054333
[5] Rezvantalab, S.; Moraveji, M.K. Microfluidic Assisted Synthesis of PLGA Drug Delivery Systems. RSC Adv. 2019, 9, 2055-2072. https://doi.org/10.1039/C8RA08972H
[6] Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of Hybrid PLGA Nanoparticles: Future of Smart Drug Delivery and Theranostics Medicine. Materials & Design 2020, 193, 108805. https://doi.org/10.1016/j.matdes.2020.108805.