Understanding the key role of Ionizable Lipids in novel RNA-lipid nanoparticle therapies
Abstract
Ionizable lipids are key enablers of recent RNA–LNP therapies and vaccines, enabling efficient encapsulation and delivery of oligonucleotide payloads through pH-dependent charge switching. This review covers their fundamental properties and introduces the latest advances in ionizable lipid design
The astonishing rapidity of COVID-19 mRNA-LNP vaccine development surprised the global community. One of the primary drivers behind this extraordinarily quick development is the adoption of an advanced drug delivery system: the ionizable lipid nanoparticle. These lipid nanoparticles have been specifically engineered for RNA delivery, with their effectiveness attributed to the inclusion of ionizable lipids in their composition. In this article, we will examine the key role played by both lipid nanoparticles and ionizable lipids and their significant influence on the development and effectiveness of a novel generation of RNA-LNP therapies and vaccines.
Lipid Nanoparticles definition
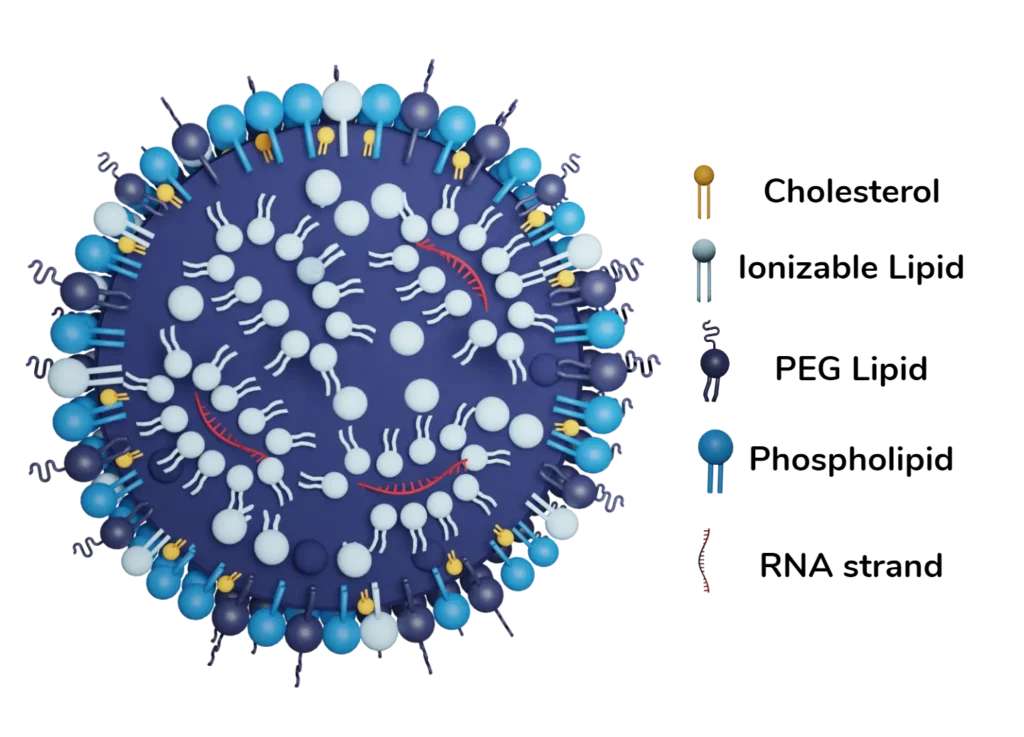
Lipid nanoparticles, also known as LNP, are an evolution of regular lipid-based nanoparticle drug-delivery systems. Generally between 50 and 200 nm in size, they are specially designed for the encapsulation and intracellular delivery of nucleic acid such as mRNA, siRNA, DNA, ASO…
In addition to their nucleic acid encapsulation capabilities, LNPs offer improved drug solubility, enhanced drug stability, and controlled release of active compounds.
Typical manufactured by microfluidics, LNP formulation comprises four essential lipid components:
- Pegylated Lipid: Serving as a protective shield, this component enhances the nanoparticle’s circulation time in the bloodstream.
- Phospholipid: The role of this helper lipid is to provide structural integrity. Those phospohlipids are integral to the nanoparticle’s membrane-like structure.
- Sterol Lipid: Offering stability, sterol lipids contribute to maintaining the structural integrity of the LNP.
- Ionizable Lipid: The innovative cornerstone of LNP, they play a pivotal role in enabling efficient drug delivery by adapting to the environmental conditions.
What Are Ionizable Lipids?
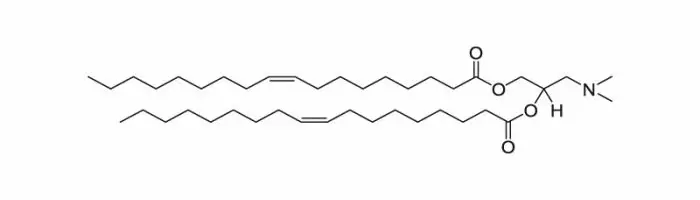
Those lipids belong to a distinctive category of lipid molecules engineered with the remarkable ability to adapt their charge in response to pH levels. Under physiological conditions, these lipids maintain a neutral charge, but as the pH decreases, they undergo ionization, ultimately acquiring a net positive charge.
This distinct characteristic sets them apart from conventional cationic lipids and provides a noteworthy advantage. Ionizable lipids harness the best of both worlds: they offer the benefits associated with regular cationic lipid, such as improved drug loading and enhanced intracellular delivery. Simultaneously, they mitigate the issue of heightened toxicity often associated with cationic lipids
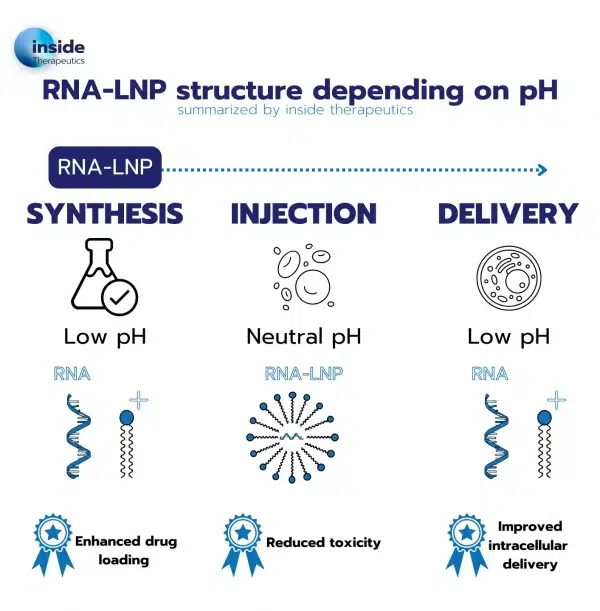
This pH-dependent charge is pivotal for three primary reasons:
- Enhanced Drug Loading: Lipid nanoparticles can encapsulate a wide range of drugs, including hydrophobic and hydrophilic compounds. When they self-assemble into lipid nanoparticles, these nanoparticles can incorporate a higher payload of drugs, thus increasing the drug-loading capacity compared to traditional lipids.
- Reduced Toxicity: Upon returning to a physiological pH environment for body delivery, the ionizable lipids composing the lipid nanoparticle return to a neutral charge state. Compared to other lipid-based nanoparticles using mere cationic lipids, this unique feature contributes to lower the overall toxicity of the system.
- Improved Intracellular Delivery: The ability to respond to pH changes is especially advantageous for intracellular drug delivery. Once taken up by cells through endocytosis, the lipid nanoparticles can undergo pH-induced structural changes, facilitating drug release within the cell’s interior through endosomal escape. This ensures efficient drug delivery to the target cell.
Insight on the types of ionizable lipids
Multiple types of ionizable lipids are available. Each of them provides a unique tradeoff, offering adaptability and innovation to RNA therapeutics developers.
To illustrate this, our article on the optimization of ionizable lipid nanoparticle for mRNA delivery shows that the right choice of LNP composition can lead to a 100 folds increase in the RNA-LNP transcription efficiency.
Unsaturated
These lipids have varying fluidity and effectiveness due to tail saturation. Increased lipid tail unsaturation improves membrane disruption and payload release. The linoleyl tail, a key component of the MC3 ionizable cationic lipid, aids hepatic gene silencing and is crucial for Onpattro®, the first FDA-approved siRNA drug. Yet, unsaturation doesn’t guarantee efficient in vivo RNA delivery, requiring careful design and screening.
Multi-Tail
Distinguished by having three or more tails, multi-tail ionizable lipids create a more cone-shaped structure, enhancing their endosome-disrupting ability due to an increased cross-sectional area of the tail region. Combinatorial chemistry aids in their synthesis, making them readily adaptable for high-throughput screening. These multi-tail ionizable lipids have been initially developed for siRNA delivery but can be repurposed for mRNA delivery. Optimized formulations of these lipids exhibit substantial increases in mRNA expression, making them invaluable for various mRNA delivery purposes, including applications like chimeric antigen receptor T-cell engineering and prenatal protein replacement therapy. Despite their impressive performance, it is essential to acknowledge that the lead multi-tail lipids often have stable backbones and low degradability, raising concerns about potential toxicity and immunogenicity.
Ionizable Polymer-Lipids
Those lipids are produced by substituting free amines on cationic polymers with alkyl tails, improving particle formation through hydrophobic aggregation. Notably, certain ionizable polymer-lipids have shown a strong ability to transfect liver endothelium and lung vasculature, suggesting a preference for endothelium. Their capabilities in transfecting different cell populations can vary based on the LNP formulation. Despite their promise, the complexity of ionizable polymer-lipids remains a challenge, as they often comprise a mixture of different substitution compounds. Furthermore, their toxic polycation core and non-degradable backbone pose potential hurdles for clinical translation.
Biodegradable
To reduce potential side effects and accumulation, biodegradable ionizable lipids are designed to be readily degraded into non-toxic metabolites following successful intracellular cargo delivery. This feature is crucial for RNA therapeutics that require repeated dosing. Various strategies, including the introduction of ester bonds, have been employed to enhance biodegradability. Ester-containing ionizable lipids have demonstrated promise, yet there’s a balance to be struck between activity and degradability. Moreover, the position and steric effects of the ester groups can significantly affect their clearance and potency. Another approach to improve biodegradability involves incorporating disulfide bonds, which are sensitive to the reductive intracellular environment. They have shown efficient delivery of ASOs and CRISPR/Cas9 systems for gene silencing and editing with good tolerability. Despite their promise, the complexity of synthesis and the risk of premature release are factors that need to be considered.
Ionizable lipid nanoparticles in practice
Usage in ongoing clinical trials
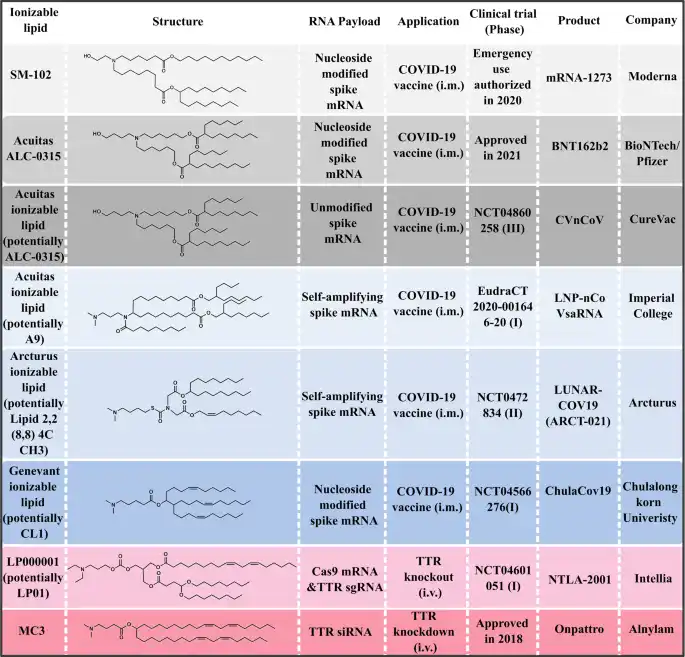
Scientists have been screening a wide range of ionizable lipids since their discovery in the 90s. In 2018, the success of the MC3, used in the Onpattro® has brought back novel ionizable lipids to the forefront for RNA delivery, greatly supporting the fast development of novel RNA-LNP therapeutics. This is best illustrated through the mRNA vaccines for which two LNP-based mRNA vaccines (mRNA-1273 and BNT162b2) were released in less than two years while demonstrating protection efficacies above 94%.
It is interesting to note that both ionizable lipids used in the vaccines (Moderna’s SM-102 and BioNTech’s Acuitas ALC-0315) share certain characteristics, such as the presence of tertiary amines, branched tails, and ester linkers. Additionally, they both exhibit extended aliphatic branches, resembling multi-tail structures. Currently, several more COVID-19 mRNA vaccines utilizing LNPs are in clinical development. While structures of the lipids used for these vaccines remain confidential, we can identify potential candidates based on existing patents and literature:
Typical applications
Ionizable LNPs have found utility in the field of nucleic acid delivery with several RNA-LNP vaccines already available. Here is a quick overview of the field where RNA-LNP therapies show the most promise:
Infectious disease vaccines
The Covid 19 vaccine is the perfect example where this innovation has proven to be game changers. These mRNA vaccines are based on the use of an ionizable lipid nanoparticle for the intracellular delivery of messenger RNA (mRNA) strand that encodes a specific protein, typically a protein found on the surface of the targeted virus. Within the cytoplasm, the mRNA is translated by ribosomes, leading to the protein expression. Consequently, the immune system learns to identify this foreign protein and generates corresponding antibodies. The adaptability of this technique renders it a distinctive tool for addressing a wide spectrum of illnesses, spanning from flu, COVID vaccine, influenza, HIV…
Cancer vaccines
mRNA LNP can also be used for cancer vaccines. In this context, mRNA vaccines are employed for therapeutic purposes, rather than for prevention. Rather than seeking to stimulate an antiviral immune response, the mRNA sequences are engineered to elicit anti-tumor immune reactions by encoding tumor-associated antigens (TAAs) or neoantigens. This adaptability enables mRNA-based vaccines to be applied across a broad spectrum of cancer types, encompassing melanoma, pancreatic cancer, brain cancer, breast cancer, and ovarian cancer.
Gene therapies
In addition to mRNA, LNPs can also be used for the delivery of other types of nucleic acids such as siRNA, or even ASO. Their unique gene-silencing abilities, by suppressing the expression of their complementary mRNA-associated gene, help slow down or even stop disease progression.
Conclusion
Ionizable lipids have become an indispensable asset in the development of lipid nanoparticles for nucleic acid delivery in the pharmaceutical industry. Their pH-dependent charge and ability to enhance RNA loading and intracellular delivery while lowering toxicity have revolutionized the field of drug delivery systems, especially in the realm of mRNA vaccines and gene therapies. As research in this area continues to evolve, the development of novel ionizable lipids for improved LNP formulation promises to bring about safer, more effective, and targeted drug delivery systems, ultimately benefiting patients and improving treatment outcomes.

Looking for the right LNP formulation solution?
Reach out to us to learn how we can help!
Reference
[1] Han, X., Zhang, H., Butowska, K. et al. An ionizable lipid toolbox for RNA delivery. Nat Commun 12, 7233 (2021). https://doi.org/10.1038/s41467-021-27493-0