Abstract
This review on LNP manufacturing provides a comprehensive overview of LNP synthesis methods, spanning from traditional and top-down techniques to advanced bottom-up nanoprecipitation approaches for lipid nanoparticle (LNP) formulation. It provides deeper insight into the most widely adopted and advanced methods—such as microfluidics—for achieving controlled, efficient, and reproducible LNP manufacturing, with precise control over particle size, PDI, encapsulation efficiency, and morphology to meet the stringent demands of drug development.
Numerous methods are available for the synthesis of Lipid nanoparticles (LNP) and more generally for drug-loaded liposomes or polymer-based nanoparticles, such as PLGA nanoparticles.
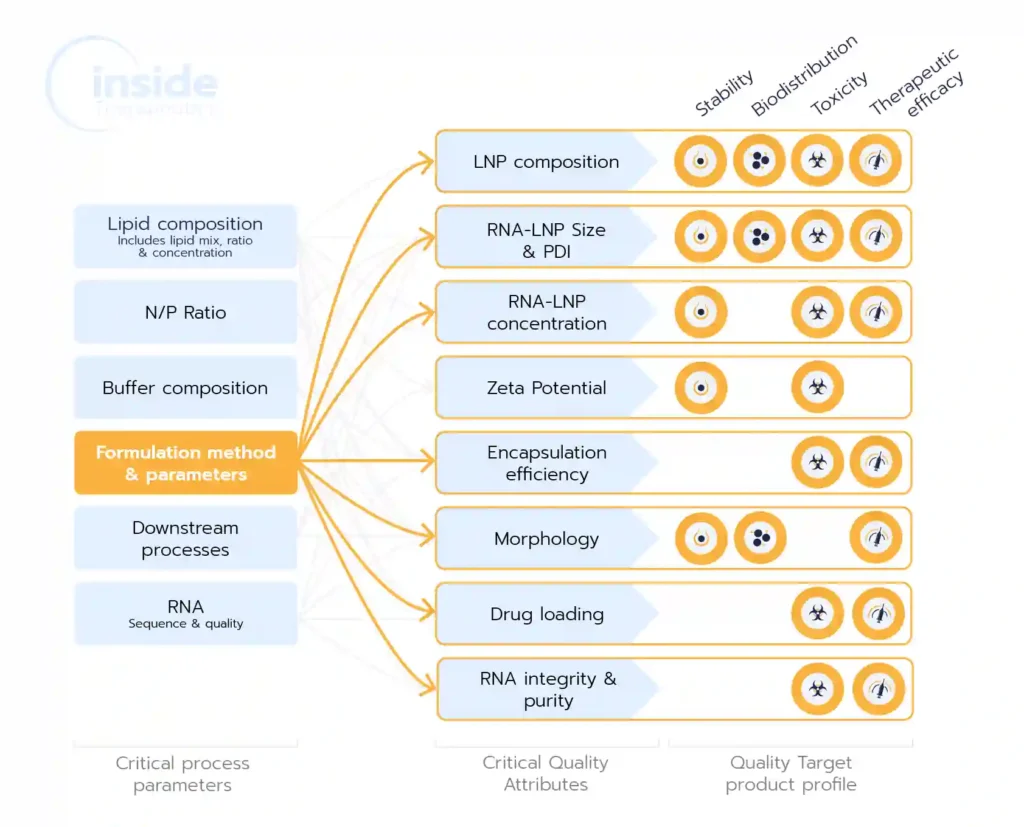
Beyond selecting reagents—such as composition, ratios, and concentrations—the choice of formulation method is crucial as it impacts nearly all the CQAs (Critical Quality Attributes) of the drug product/final physicochemical properties, such as particle size, encapsulation efficiency, encapsulation yield, and morphology, which in turn, play a critical role in determining pharmacokinetics, cellular uptake, and eventually overall drug efficacy, toxicity, biodistribution..
Therefore, it is essential to carefully choose the formulation method based on factors such as the intended route of administration, target biodistribution, and the development stage of the molecule to ensure the most efficient drug development.
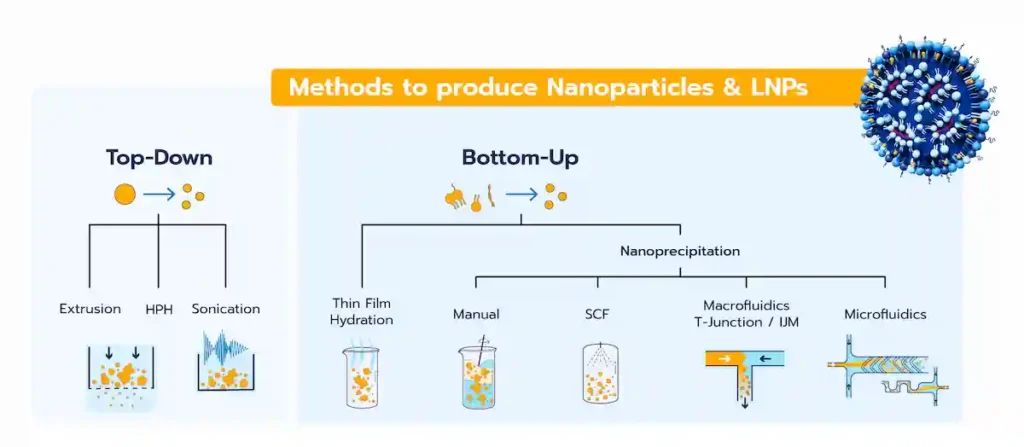
The LNP manufacturing methods, and more generally all the lipid-based nanoparticles, can be categorized into 2 different classes:
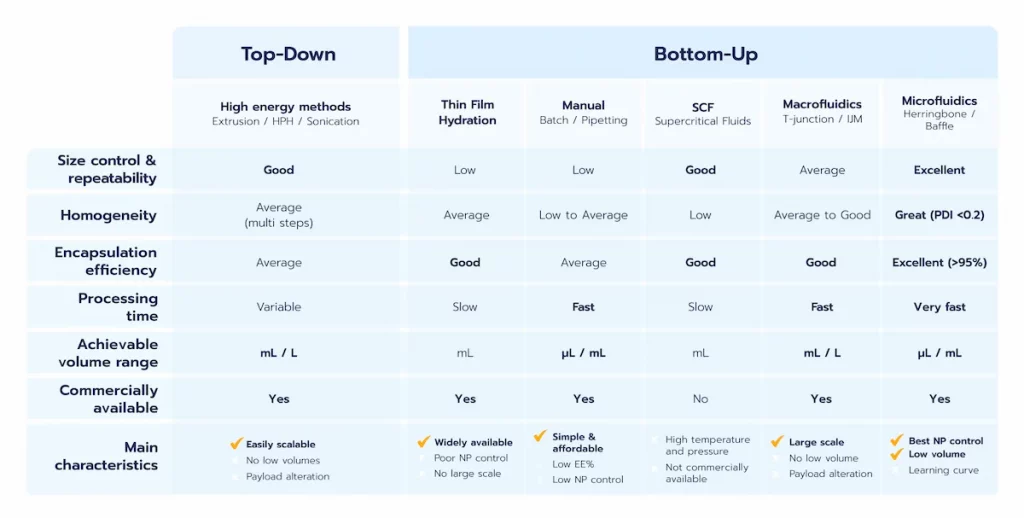
- Top-down methods: Also referred to as high-energy methods, these involve applying energy to break larger particles into smaller ones.
- Bottom-up methods: These rely on the self-assembly of lipid components to form homogeneous nanoparticle systems.
This article aims at providing an introduction to those formulation processes, from the historical ones, to the state of the art.
Why the LNP Formulation Process is critical for developing successful drug products ?
The development of successful lipid nanoparticles (LNPs) depends on a combination of factors, including the selection of appropriate lipids, their ratios, and concentrations. However, the formulation process itself is equally critical, as it directly shapes the Critical Quality Attributes (CQAs) of the nanoparticles—such as particle size, polydispersity index (PDI), encapsulation efficiency, yield, and morphology. These CQAs, in turn, play a pivotal role in determining the drug product’s pharmacokinetics, cellular uptake, toxicity, and overall therapeutic efficacy.
Given this cascading impact—from nanoparticle properties to clinical outcomes—the choice of the formulation process is a strategic decision that determines the entire drug development pipeline. An optimized formulation method not only ensures the production of high-quality LNPs but also minimizes risks associated with suboptimal formulations, such as inconsistent performance or complications during LNP Formulation scale-up. Consistency and reproducibility across scales are vital for regulatory compliance and successful clinical translation, making the formulation process a cornerstone of effective drug development.
Drug development process steps and requirements
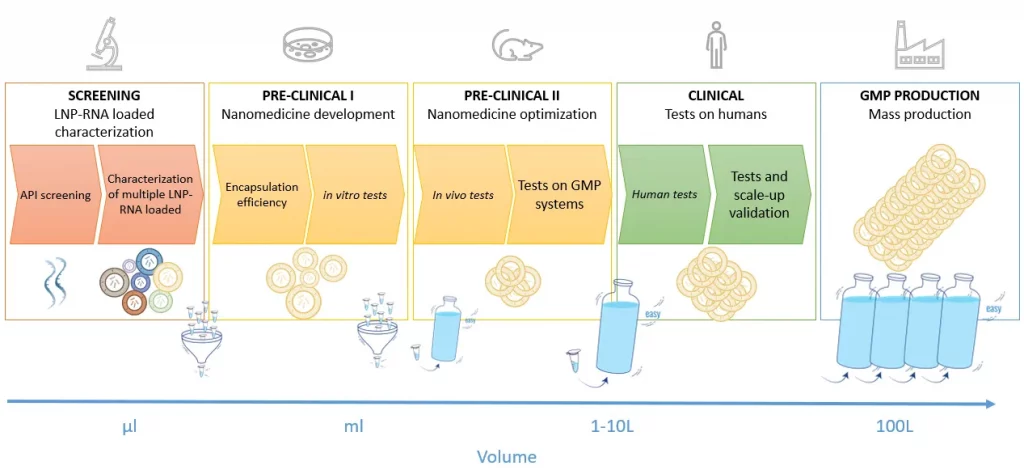
To start with, it is important to bear in mind that the lipid nanoparticle manufacturing requirements vary throughout the drug development process, as described in figure 1.
Whether it is for mRNA vaccine for infectious diseases or cancer, gene therapy or mRNA therapeutics, the initial steps of the process require the test of many different RNA LNP complexes (up to several 100s) with different RNA, lipids mix, and synthesis conditions… to find the most optimal one. It has indeed been proven that optimizing the nanoparticle delivery vessel can lead to a 100-fold improvement of the final mRNA transcription.
Due to the very high cost of lipids and RNA, working with a very low volume (100s of µL) of RNA-LNP is required at that stage. Depending on the chosen formulation technology, the manufacturing time can also be lengthy, which can also make the tests of hundreds of LNPs very painful.
As the process moves forward, the number of lipid nanoparticle formulations shrinks down (generally 10s of the in-vivo tests and 1 for the Mass production), while the volume requirements greatly increase, up to 100s of L. The ideal LNP manufacturing process should thus allow for the seamless transition from the initial LNP screening phase at very low volumes, all the way to the drug mass production process by keeping the RNA LNP complex as identical as possible at every stage of the development process.
Finally, as the development process progresses closer to the human trials and production, it is required to take into account current good practice manufacturing (cGMP manufacturing or GMP manufacturing) to ensure meeting the pharmaceutical industry guidelines and allow for a quick and smooth transition to market of the drug product.
Top-Down methods for LNP synthesis
Top-down approaches are generally high energy methods that rely on the generation of high shear stress forces by putting the lipids into harsh conditions (high pressure, high temperature) to achieve LNP size reduction.
The goal of these technics is to provide sufficient energy to the particles in the system to fragment them. The higher the surface tension the more energy should be supplied to the system to break them. These approaches be used either by themselves to produce nanoparticles from bulk material (such as illustrated below) or in combination with another low-quality method, to refine and improve the quality of nanoparticle populations. A typical combination is the use of thin film hydration methods together with an extruder.
Overall, those methods offer the advantage of being already available at a large scale. However, the high energy involved in the system can lead to lipid or nucleic acid alteration, thus not making is sustainable for biological application. On top of this, they offer poor encapsulation efficiency and struggle with low volume formulation (< mL)
High-pressure homogenization (HPH)
High-pressure homogenization (HPH) permits the creation of an emulsion of molten lipids (generally 5-10%), water, and surfactants using a high-speed stirrer. The solution is pushed at high speed through small orifices using high pressures (500 to 5000 bar). The high velocities reached by the fluid are accompanied by turbulence, high shear forces, and cavitation which allows the formation of organic NPs even at high concentrations.
Two main approaches are available for LNP synthesis with HPH: hot and cold homogenization.
Cold Homogenization
For cold homogenization, the drug-lipid melt is cooled down to form microparticles. The particles are then dispersed in a cold surfactant to generate a presuspension. The presuspension is then homogenized to break down the particles and form the nanoparticles. This approach permits the work with thermosensitive drugs by minimizing the diffusion of hydrophilic drugs into the water phase.
Hot homogenization
In hot homogenization, a drug and lipid mix is brought to a temperature above their melting point. The mix is then dispersed into a hot surfactant while maintaining the temperature. A hot pre-emulsion is formed, which is then homogenized before being cooled down, allowing the formation of the nanoparticles.
Ultrasonic homogenization
LNP can also be manufactured using ultrasounds. With this method, ultrasound waves will create cavitation phenomena, leading to the formation and collapse of bubbles, and thus the synthesis of LNP. In addition to this,the energy brought to the system helps breaking the nanoparticles, achieving size reduction. While offering size control, this method offers very low encapsulation efficiency and can lead to lipid degradation as well as metal pollution. [1]
Bottom-Up approach for LNP formulation
Unlike top-down methods, the bottom-up approach focuses on starting with the basic components of nanoparticles, such as lipids or polymers, and assembling them into the final nanoparticle product. This approach includes several techniques, such as thin-film hydration or a broad range of nanoprecipitation/self-assembly methods.
Thin film hydration
The Thin Film Hydration Method, also known as the Bangham method, is the historical technique used for lipid nanoparticle (LNP) and liposome formulation. The process involves dissolving lipids in organic solvents, creating a thin lipid film via solvent evaporation, and hydrating it with an aqueous solution to trigger nanoparticle formation.
However, due to the poor control of their synthesis/mixing condition, the physicochemical properties of the LNP manufactured are very poor with a very low batch-to-batch reproducibility, and very high sample size dispersity, and near no control of the size … For these reasons, Thin Film Hydration approaches are generally combined with high energy technics to improve the level of control over the nanoparticles formulated.
Nanoprecipitation-based methods for LNP synthesis
Methods based on self-assembly, also known as nanoprecipitation, constitute the primary approach employed in the development of the LNPs involved in the Covid-19 vaccine.: The underlying principle is mixing an aqueous phase, containing the hydrophilic API or oligonucleotide to be encapsulated, with a water-miscible solvent, such as ethanol, containing the lipids.
The decrease in the partial concentration of the solvent when mixed with the aqueous phase will lead to a drop in solubility of the lipids, which will then triggers its self-assembly and grow to create nanoparticles.
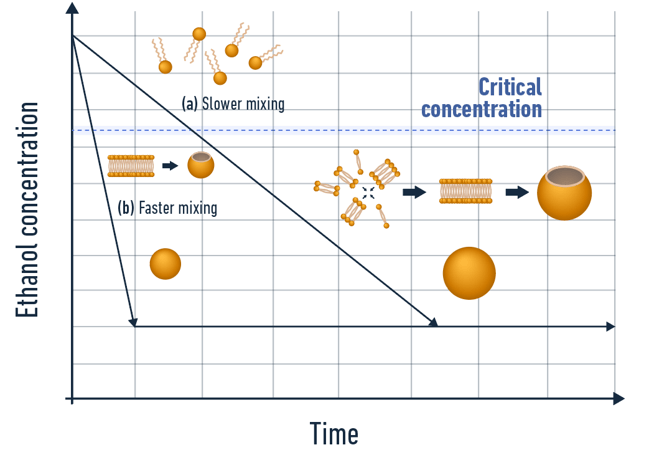
The self-assembly process is divided into 4 steps supersaturation, nucleation, growth, and stabilization or maturation. As introduced in our lipid nanoparticles formation review, the physicochemical characteristics of the formulated LNP are highly dependent on the synthesis conditions.
The most important factors are:
- The faster the mixing, the smaller the synthesized LNP
- Increasing the concentration of stabilizing agents decreases the size of the manufactured LNP
- Higher concentration of solute leads to a larger number of nucleic, hence an increase in coalescence events and final size of the LNP
- Viscosity and temperature play a role but their influence on the manufactured LNP
The main limitation of the solvent-based methods is the solvent potentially remaining in the final solution. However, the regulatory framework is relatively flexible in terms of ethanol content (up to 0,5% for the FDA), the remaining excess solvent can easily be extracted using centrifugation, Tangential flow filtration, Dialysis…
In addition to this, the post process purification step also ensured better stability of the final drug product.
Manual & Batch methods
Manual methods, such as pipetting, or larger scale ones in tanks can be used for the formulation of LNP using nanoprecipisation. However, the poor control over the mixing conditions of these methods leads to poor control and repeatability over the final nanoparticle characteristics. They are therefore a good way to carry out some preliminary proof of concepts, but should be quickly replaced by more reliable & repeatable methods such as desribed below.
Supercritical Fluid Methods (SCFs)
SCFs methods have been developed to minimize the use of solvent in the nanoprecipitation process.
Several methods are available, the most popular one being the Supercritical Anti-solvent Method (SAS). A supercritical fluid is passed through the pressurized chamber, while the drug is sprayed on the SCF using a nozzle. LNPs are generated when hydrated with the aqueous solution. The supercritical CO2 is miscible with the organic solvent and acts as an antisolvent to the solute.
However, when put in practice, those methods suffer from major drawbacks: First, they can only process principles that are soluble in scCO2, which greatly limits them. Also, the high pressure/high temperature generally involved leads to risks for the API. In addition to this, those methods require very high investment cost and high energy consumption, which makes them hard to implements at large scale. Finally, due to their complexity, those protocol are not currently commercially avaialble.
Impingement jet mixing/T-junction
As a first step to enhance nanoprecipitation,T-mixers, such as impingement jet mixers, have been developed. Those systems are based on flash nanoprecipitation, where the solvent and anti solvent phase are mixed at very high speed and mix nearly instantly. While being efficient for large batches – such as used in the covid 19 vaccine – those methods require large volumes of liquids (at least several mL) and are thus not suitable at early stage of the drug development process. Additionally, they combine some of the drawbacks of high energy methods due to the large pressure involved, which can lead to API degradation.
State of the art: LNP synthesis using microfluidics
Considering the importance of finely controlling the mixing parameters to obtain homogenous LNP populations and good batch-to-batch reproducibility by nanoprecipitation, the use of microfluidic quickly appeared as an evident solution to synthesize LNP.
Microfluidics is the technology of manipulating small volumes of liquids, down through nL, through micro-channels. At the micrometer scale, fluids behave differently than in everyday life and show laminar flow condition, which permits excellent control of their flow behavior all throughout the synthesis process, as well as provide an increased volume/surface ratio. On top of this, the ability of microfluidics to handle very low volume formulation – critical for a cost efficient screening – makes it the ideal solution for the first development stages of a RNA-LNP drug product.
At that scale, thanks to the laminarity of the fluidic flow, the mixing process of the solvent and aqueous phase, triggering the self-assembly of the nanoparticle, offers the main advantage of being extremely well controllable and reproducible.
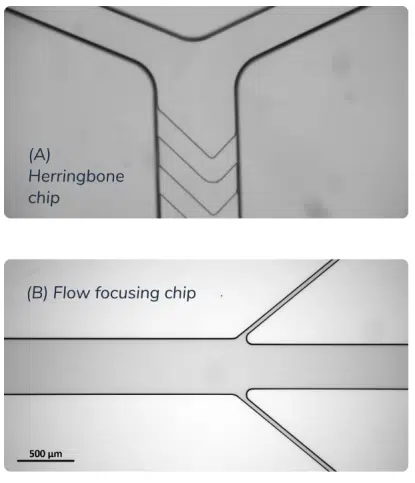
Multiple mixing methods using microfluidics are available. The most popular ones are the diffusive method (such as flow focusing), the chaotic methods (herringbone or the baffle mixer). They all provide different achievable flow rate, efficiency, repeatability, encapsulation efficiency but overall offer better trade-offs than all the previously mentioned LNP synthesis process, and are currently considered the state-of-the-art LNP formulation method.
Conclusion on LNP manufacturing
In conclusion, the manufacturing of lipid nanoparticles (LNPs) encompasses a range of methods, from traditional approaches like high pressure homogeneizers and thin-film hydration to cutting-edge technologies such as microfluidics, each with distinct advantages and limitations. The choice of formulation method is critically influenced by the stage of drug development, intended application, and specific requirements such as particle size, encapsulation efficiency, batch reproducibility, and scalability.
During early-stage screening, methods that support low-volume production and offer precise control over nanoparticle characteristics are essential, to decrease cost with no comproise on quality. Microfluidics has emerged as the gold standard in this phase, providing unmatched reproducibility, fine-tuned control over synthesis conditions, and adaptability for small-scale formulation testing. However, due to the inherent constraints of microfluidics, including clogging and low throughput, scaling up and accomodating large volumes can be challenging and may require complementary processes.
For large-scale production, high-energy methods such as high-pressure homogenization or impingement jet mixing (T-junctions) are commonly employed, as they accommodate larger volumes efficiently. While these methods may trade off some degree of precision compared to microfluidics and cannot access very low volume formulation, making them not suitable for screening, they are well-suited for meeting the demands of mass production.
Ultimately, selecting the most appropriate LNP formulation method necessitates a comprehensive understanding of the intended application, the physical and chemical properties of the LNPs, and the regulatory and practical requirements of the production process. As outlined, a seamless transition between early-stage development and large-scale manufacturing remains a critical challenge. Addressing this gap will require novel technical innovation to enable a more streamlined and efficient LNP development pipeline.
Characterization of the manufactured LNP
As highlighted in the introduction, the choice of manufacturing method during the LNP formulation process significantly influences all critical quality attributes (CQAs) of the nanoparticles. This section explores the most essential CQAs and examines how formulation conditions affect these attributes.
Lipid nanoparticles have physicochemical attributes that need to be precisely controlled all throughout their synthesis process in order to reach the targeted area and achieve the desired effects in the body,
There is no strict regulatory framework for nanomedicines and no quantitative targets are set by the competent authorities, namely the European Medicine’s Agency (EMA), the Food and Drug Administration (FDA), and the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH).
However, guidelines exist and require a careful characterization of the physicochemical attributes of the nanomaterials used in a drug formulation.
These attributes include the size of the NPs, the polydispersity index (PDI) associated with their size distribution, their morphology, and internal structure as well as their surface charge. Every batch of NPs needs to be properly characterized before proceeding to in vitro or in vivo assays. Quality checks are also needed in large-scale production of drugs or vaccines based on LNPs. Go deeper on the topic in our LNP and liposomes characterization review.
Size and distribution characterization following LNP synthesis
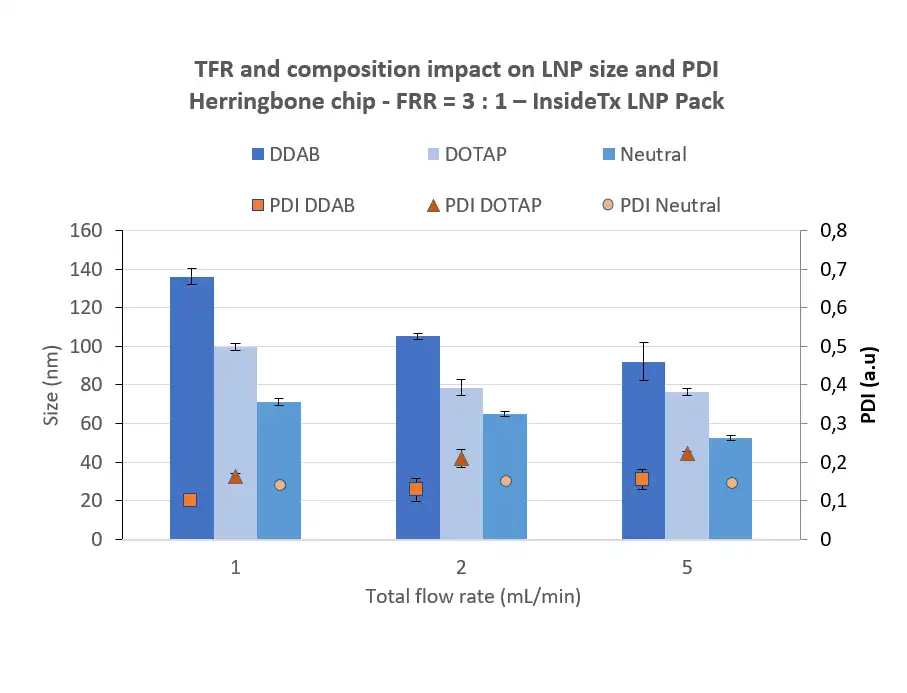
The lipid nanoparticle size is crucial to determine its in vivo release.
For optimal mRNA delivery, a compromise should be found between decreasing the particle size, which allows for easier absorption to the cell, and the LNP toxicity which increases when the particle size gets too small. Most ionizable lipid nanoparticles used are currently in the 50 to 200 nm range, with an optimal around 100 nm for LNP.
The particle size distribution characterization, or “polydispersity index” (PDI), defines the size range of lipid nanoparticles. This index is dimensionless and is scaled so that PDI <0.05 are nearly homogeneous, and PDI >0.5 are totally heterogeneous.
Typically, PDI below 0.2 is sought for the population of lipid nanoparticles and can easily be obtained using microfluidics methods.
It is worth noting that counterintuitively, Lipid nanoparticle size is mostly driven by the lipid mix and the manufacturing condition rather than the size of the RNA cargo.
The synthesized LNPs are generally characterized using optical methods such as dynamic light scattering (DLS) or laser diffraction (LD).
LNP surface charge – Zeta potential characterization
The Zeta potential provides information on the charge at the surface of the LNP. It helps predict the long-term stability of the formation. In order to avoid aggregation, it is necessary to have a zeta potential value as high as possible in absolute value (|ζ| ≫ 30mV). Indeed, when the latter tends towards 0 (-10 mV ≤ ζ ≤ 10 mV, the inter-particle forces decrease, and the attractive Van der Walls forces become preponderant. Generally, zeta potential values in the order of -30 mV are reported to both avoid the aggregation and decrease toxicity.
Zeta potential can also be characterized using specific features of a DLS, or laser Doppler electrophoresis.
LNP surface morphology characterization
Surface morphology is generally characterized using microscopy methods. Most often Scanning electron microscopy (SEM), Transmission electron microscopy (TEM) or Atomic Force Microscopy (AFM) are used.
Each of the methods offers a specific compromise. TEM provides a 2D image of the sample and gives access to the internal structure of the LNP with sub nm resolution. AFM instead permits a 3D visualization of the sample, including its surface with no specific sample preparation.
Load capacity (DL)/Encapsulation Yield (EY%) and Encapsulation Efficiency (EE%)
These parameters are crucial for assessing the ability of lipid nanoparticles (LNPs) to efficiently encapsulate the active pharmaceutical ingredient (API).
- Drug Load (DL%): This parameter represents the proportion of API encapsulated within the LNP relative to the total weight of the nanoparticles. It provides insight into the LNP’s capacity to carry the therapeutic payload.
- Encapsulation Yield (EY%): Encapsulation yield refers to the percentage of the initial API successfully encapsulated within the LNPs. It is calculated as the amount of encapsulated API relative to the total API used during the formulation process, reflecting the efficiency of the overall formulation process.
- Encapsulation Efficiency (EE%): Encapsulation efficiency measures the proportion of API encapsulated in the LNPs relative to the total API present in the final formulation. Unlike encapsulation yield, EE% accounts for losses that occur during the formulation process, such as API degradation or waste. For RNA-loaded LNPs, EE% is particularly critical, as excessive free RNA can increase toxicity and compromise therapeutic outcomes.
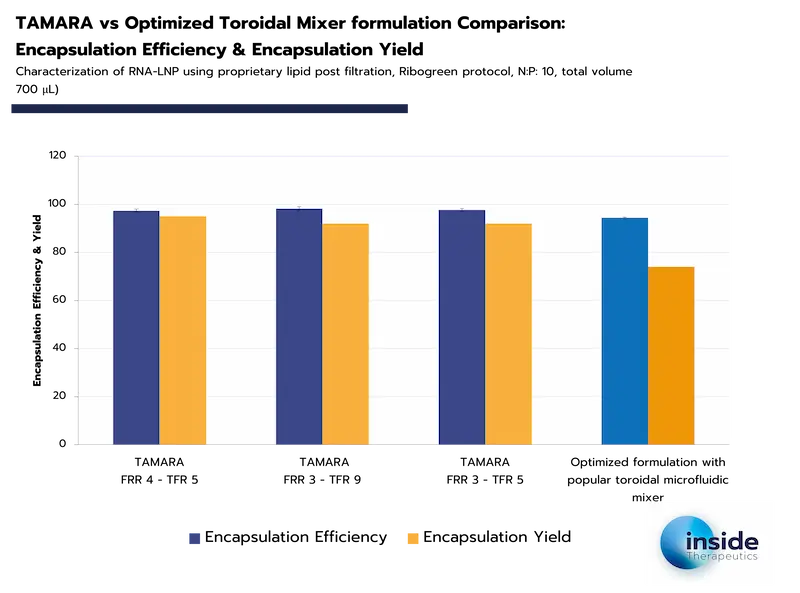
Encapsulation efficiency is highly dependent on the synthesis method used. Advanced techniques such as microfluidics can achieve encapsulation efficiencies exceeding 90%, highlighting their superiority in ensuring both high API loading and minimal loss during formulation.
In contrast, encapsulation yield reflects not only the method’s efficiency but also operational losses from the synthesis equipment, as shown in the examples below. Carefully selecting not only the formulation method but also the machine itself therefore appears event more critical.
General conclusion on LNP formulation
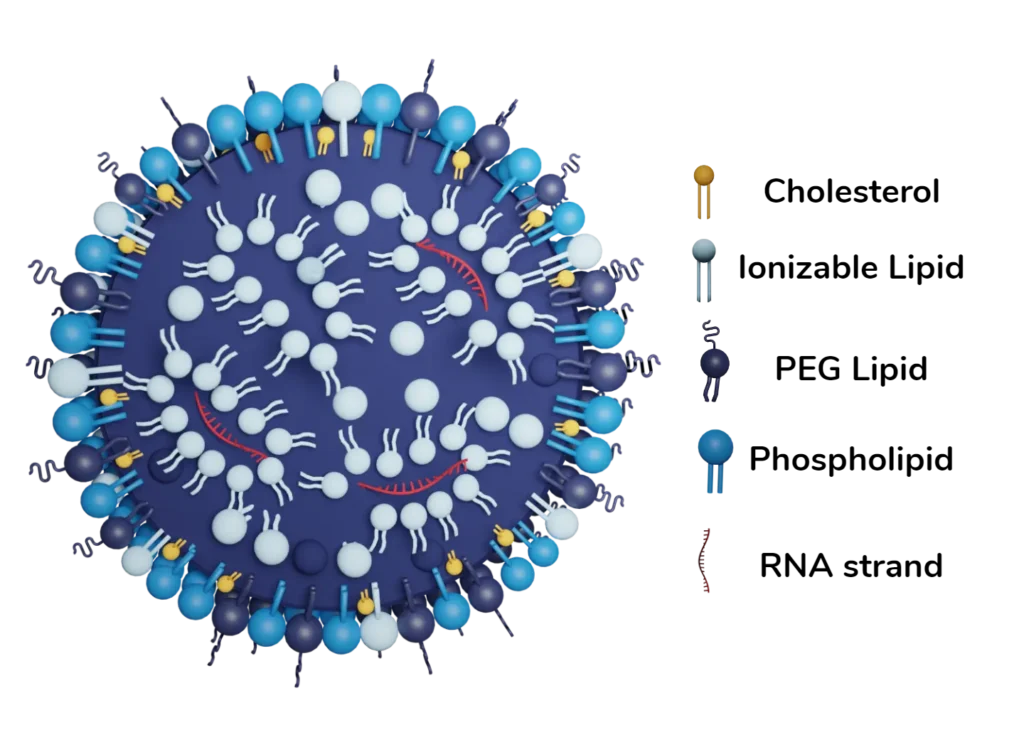
Lipid nanoparticles (LNP) are a promising new non viral delivery system for nucleic acid, such as mRNA, siRNA, saRNA, and DNA. Indeed, LNP are able to safely and effectively carry out the intracellular delivery of those nucleic acids, making them an ideal candidate for the development of new vaccines and gene therapies.
However, the CQAs (size, PDI, EE%…) of the LNP are critical to their efficient delivery, and are highly influences by the formulation process used. For this reason, manufacturing methods providing good control over these critical parameters should be prefered.
Thanks to its unique ability to finely control final LNP characteristics, and its ability to work with very small volumes, microfluidics is considered the state of the art manufacturing method.
Nevertheless, the manufacturing of LNP is a rapidly developing field, and new and improved methods for manufacturing LNP are being developed all the time, so stay tuned!

Looking to get started with RNA-LNP formulation?
Reach out to us to learn how we can help!