Abstract
Nanomedicine addresses limitations of conventional medicine through nanoscale engineering, enabling targeted drug delivery, advanced imaging, and novel vaccines such as COVID-19 mRNA platforms. This review outlines various nanoparticle types, historical milestones, key applications, manufacturing strategies, ongoing challenges, and future directions, highlighting nanomedicine’s transformative potential in precision and personalized healthcare.
Nanomedicine represents a transformative approach to diagnostics and therapeutics, leveraging the unique properties of materials at the nanoscale. Its roots extend back to the mid-20th century when the concept of using nanometer-sized particles for medical applications began to materialize. The field has grown significantly over the decades, offering novel solutions in drug delivery, imaging, and diagnostics.
What is nanomedicine?
Nanomedicine: A definition
The word nanomedicine is the combination of 2 words: ‘nano’ which refers to something very small in ancient greek, and medicine “ The science or practice of the diagnosis, treatment, and prevention of disease”. Nanomedicine therefore involves the application of nanotechnology to develop innovative applications, specifically nano-objects, nanomaterials or nanoparticles in the field of health, by harnessing the physical, chemical, and biological properties of materials at the nanometric scale.
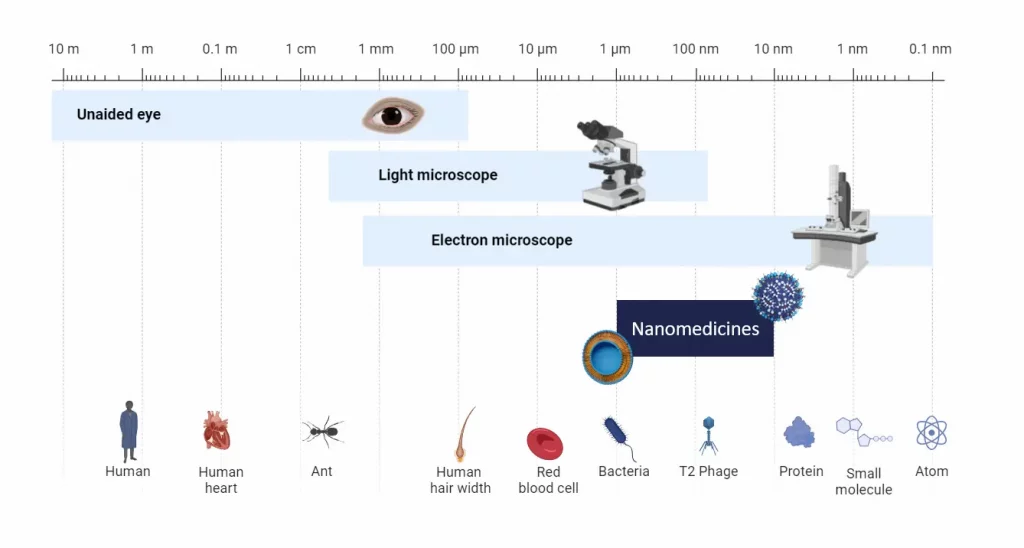
Nanomedicine scale
Nanomedicine primarily operates at the nanoscale (10-6 to 10-9 m or 1 to 1000 nm) where materials generally exhibit properties that differ significantly in physics, chemistry, or biology compared to their macroscopic counterparts.
At the nanoscale, the surface-to-volume ratio is substantially higher than at larger scales, enhancing the efficiency of particle functionalization through coatings. This process improves biocompatibility and facilitates more selective binding to specific targets.
Furthermore, the nanometer scale enables direct interaction with biological molecules such as DNA, RNA, and proteins within specific organs or cells, paving the way for innovative therapeutic strategies.
Nanomedicine types and composition
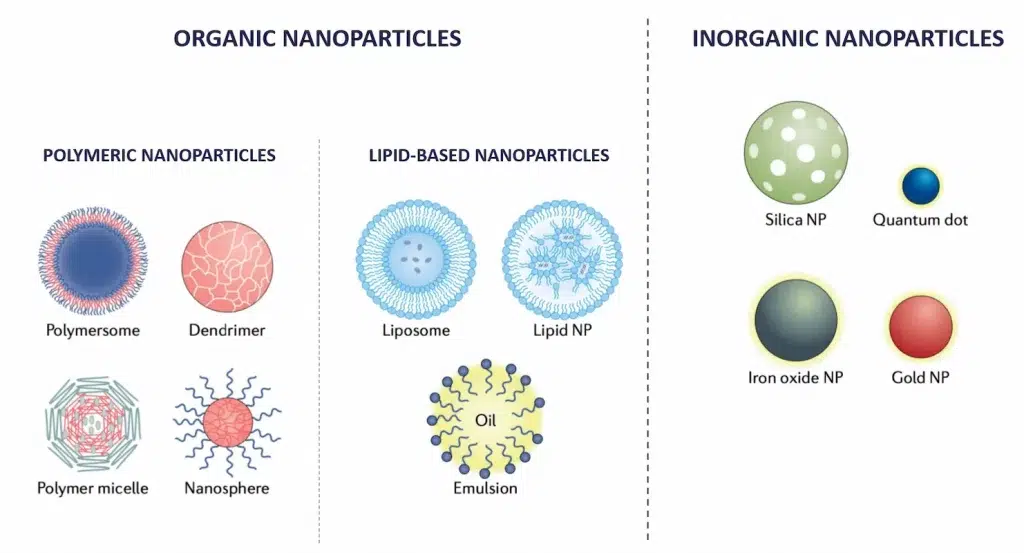
Nanoparticles in nanomedicine are typically classified based on their structure and composition into two primary categories: organic and inorganic. Each type possesses distinct characteristics and offers specific applications.
Organic nanoparticles
Organic nanoparticles are primarily made from carbon-based compounds and naturally occurring polymers. These include:
- Lipid-based nanoparticles: This category includes a broad range of lipid-based nanoparticles such as Liposomes and Solid Lipid Nanoparticle (SLN) or Lipid Nanoparticle (LNP) These spherical vesicles can encapsulate drugs, optimizing the delivery of therapeutic agents to specific sites, even within the cells, while reducing toxicity and side effects.
- Polymeric Nanoparticles: Made from biodegradable polymers, these nanoparticles are used for controlled drug release. They degrade over time, releasing the drug in a controlled manner.
Inorganic nanoparticles
Inorganic nanoparticles are typically composed of metals, metal oxides, or other inorganic materials. They are known for their robust physical and chemical properties, including:
- Optical Properties: Many inorganic nanoparticles, like gold nanoparticles, silica nanoparticles and silver nanoparticles, exhibit unique optical properties due to surface plasmon resonance, which can be utilized for imaging and diagnostic purposes in medicine.
- Magnetic Properties: Iron oxide nanoparticles, for example, are used for magnetic resonance imaging (MRI) as contrast agents due to their superparamagnetic properties.
- Thermal Properties: The ability of some inorganic nanoparticles to convert near-infrared light to heat can be used in therapeutic applications like photothermal therapy, where they help in the ablation of cancer cells.
Historical Development of Nanomedicine
When was nanomedicine invented?
The journey of nanomedicine from ancient uses to its pivotal role in modern healthcare illustrates a blend of history, science, and technology. This journey has been significantly influenced by the evolution of molecular biology, highlighting key biomolecules like DNA, RNA, and peptides. The fragile nature of these biomolecules necessitated innovative delivery systems, setting the stage for nanomedicines to revolutionize medical treatments and drug delivery. The ancient Romans, unaware of the science behind it, first used nanoparticle technology in artifacts like the Lycurgus Cup, which displayed color-changing properties due to gold and silver nanoparticles. However, the formal scientific exploration of nanoparticles began much later, around the 1950s and 1960s, marking the modern era of nanotechnology and nanomedicine.
Early Developments and the Discovery of Liposomes
One of the groundbreaking advancements in this field was the discovery of liposomes by Alec Bangham in the 1960s. Liposomes, essentially spherical vesicles consisting of one or more phospholipid bilayers, revolutionized the concept of drug delivery systems. Their ability to encapsulate active substances significantly improved the targeted delivery of therapeutic agents, reducing toxicity and enhancing efficacy. This innovation paved the way for further research in targeted therapies, particularly in the treatment of cancer and genetic diseases (Bangham, 1960s).
Advancements in Genetic Understanding and Controlled Drug Release
Simultaneously, significant strides in understanding genetic regulation were made by researchers like Jacques Monod and François Jacob at the Institut Pasteur1. Their research laid the foundational principles of genetic engineering, which later influenced nanomedicine, allowing for the development of therapies that could specifically target genetic defects at the molecular level. Their contributions were recognized with the Nobel Prize in Medicine in 1965
During the same period, Professor Peter Paul Speiser at ETH Zurich was instrumental in developing technologies for controlled and sustained drug release, including the first nanoparticles for drug delivery and vaccination2. His research aimed at reducing the frequency of drug administration, enhancing the efficiency of vaccines like those against tetanus and diphtheria
1980s: The Emergence of nanomedicine Applications and the Development of Second-Generation nanoparticles
The 1980s marked a critical advancement in nanomedicine, largely propelled by the pioneering research of Patrick Couvreur and colleagues. Their work demonstrated that nanoparticles could be effectively used for cancer therapies by leveraging the Enhanced Permeability and Retention (EPR) effect, which concentrates nanoparticles at tumor sites (Couvreur et al., 1986; Maeda and Matsumura, 1989). In parallel to this, another key application of nanoparticles in drug delivery emerged: facilitating the traversal of therapeutic agents across the blood-brain barrier (BBB).
Towards the late 1980s, a second generation of nanoparticles was developed, incorporating Poly(Ethylene glycol) (PEG) into their formulations. Earlier uncoated nanoparticles, were indeed rapidly cleared by the immune system’s macrophages. PEG-coated nanoparticles could instead evade immune detection—a phenomenon known as the ‘stealth effect’—thereby prolonging their circulation time within the bloodstream, and thus improving therapeutics effect.
1995: FDA-approval of doxyl
The approval of Doxil in 1995 represents a landmark event in the field of nanomedicine, as it was the first FDA-approved nanomedicine product made available on the market. This liposomal formulation of doxorubicin encapsulates the chemotherapy agent within lipid bilayers, pioneering the use of nanotechnology to target drug delivery directly to tumor sites while shielding healthy tissues from exposure. This targeted approach not only enhances the effectiveness of the treatment but significantly mitigates the systemic side effects commonly associated with traditional chemotherapy regimens.
2000s: the rise of nanomedicine
At the dawn of the 21st century, nanomedicine began to take a definitive shape, merging the potential of nanotechnology with medical applications to address complex challenges in the diagnosis and treatment of diseases…
The 2000s opened numerous doors for the use of nanomedicine in a much broader range of application, spanning from advanced imaging techniques to the molecular repair and manipulation of tissues.
During this period, the advancement of novel organic nanoparticles continued to revolutionize drug delivery systems. For instance, the introduction of Solid Lipid Nanoparticles significantly improved drug delivery efficiency and increased the bioavailability of medications. This advancement fueled optimism for more effective management of complex diseases, including cancer, neurodegenerative disorders, and cardiovascular diseases.
2020s: The crucial role of nanomedicine in developing COVID-19 vaccines
The 2020s witnessed the widespread recognition of nanomedicine, largely due to its indispensable role in the development of COVID-19 vaccines. LNP, those lipid-based nanoparticles specifically designed for the intracellular delivery of RNA, emerged as a critical component in the COVID-19 vaccines produced by Pfizer and Moderna.
These LNPs were essential for protecting the RNA from immune destruction and facilitating its successful entry into cells. Without this technology, the RNA payloads would likely have been neutralized before they could reach their target inside the cell, rendering the vaccines ineffective.
What are nanomedicines used for?
Nanomedicines prove to be very precise due to their ability to interact specifically with tissues, cells, and even molecules. At the “nano” scale, some substances or materials can change properties, becoming more resistant, more reactive, etc. They are thus used for a variety of applications ranging from therapeutics, and diagnostics to more innovative theragnostic approaches.
Nanomedicine for the development of novel therapies
Drug delivery systems
The development of novel nanoscale drug delivery systems has been a major step forward for therapeutic strategies. These systems help in enhancin the delivery and efficacy of drugs while significantly reducing toxicity and side effects.
Nanomedicine has introduced the development of novel nonviral delivery methods using nanoparticles, including liposomes, PLGA nanoparticles or LNP, to transport and deliver API (active pharmaceutical ingredient) directly within the targeted cells enhancing bioavailability both spatially and temporally.
The efficacy of these drug delivery through nanomedicine is largely based upon efficient encapsulation of the API and its successful delivery to the targeted site. This is made possible by the nanoparticles’ inherent ability to traverse cellular membranes, thereby optimizing cellular uptake. The enhanced permeability and retention (EPR) effect further aids in accumulating these therapeutic agents near tumor cells without systemic exposure, reducing potential side effects.
Several nanomedicines have already made their mark on the market, illustrating the substantial benefits of nanotechnology in drug delivery. A prime example is the liposomal formulation of doxorubicin. Studies have shown that this nanomedicine format not only maintains the drug’s efficacy but also ameliorates associated toxicities, such as cardiac risks and gastrointestinal side effects like nausea and vomiting, compared to its traditional counterpart.
Current research is dynamically focused on the next generation of nanoparticles, aiming to enhance nanoparticle targeting capabilities further. This involves two primary strategies:
- Passive Targeting: Refinement of nanoparticles’ physicochemical properties, such as size and composition, to improve their stability and specificity.
- Active Targeting: Functionalization of nanoparticles’ surfaces with specific ligands or antibodies to facilitate direct interaction with target cells or tissues. This approach aims to minimize toxicity and prevent off-target effects by ensuring that the nanoparticles accurately locate and adhere to their intended targets.
Novel vaccine development
In addition to improved delivery of API, nanomedicines have allowed for the development of a whole novel range of therapeutics involving the use of nanometric-sized biological elements, such as DNA, RNA, and peptides to treat diseases.
Combined with the novel non-viral drug delivery system this allowed for the to open new possibilities for development therapies.
The development of COVID-19 mRNA vaccines is a standout example of nanomedicine’s impact. In these vaccines, mRNA coding for the SARS-CoV-2 spike protein was injected into the human body encapsulated into an LNP. The mRNA was then introduced to the cell before being translated into the corresponding protein, thus optimizing the body’s defensive response to the virus. Additionally, as a special type of lipid-based nanodrug delivery system, the Lipid nanoparticles (LNPs), have been crucial in the mRNA vaccine development. They helped protect the mRNA from degradation, facilitate its entry into host cells, and thus boost the vaccine’s effectiveness.
Diagnostic Tools: Use of nanoparticles in imaging and early disease detection
The advancement of nanomedicine has greatly influenced the field of diagnostic imaging, especially with the use of nanoparticles specifically desgined to enhance the visualization of diseases. Preclinically, nanoparticles labeled with contrast agents have proved invaluable for understanding the dynamics of drug delivery systems, providing insights into circulation properties, target site accumulation, and off-target effects. These non-invasive imaging techniques facilitate the monitoring of drug release and therapeutic efficacy over time.
However, the clinical application of nanodiagnostics remains limited due to strict pharmacokinetic and elimination criteria required for intravenously administered diagnostic agents. While nanodiagnostics have found some utility in clinical settings—such as imaging tumor vasculature, labeling stem cells, and identifying liver lesions—their general use is constrained. Nanoparticles often face challenges in efficiently penetrating and distributing within targeted sites due to their size and prolonged circulation times. Moreover, their large size and the non-specific accumulation often hinder the differentiation between targeted and passive accumulation, affecting diagnostic accuracy.
Despite these limitations, specific large imaging agents like microbubbles and nanobubbles show promise in clinical trials for targeting vascular structures in tumors, offering high signal-to-background ratios due to their size and short circulation times. These developments highlight the ongoing need for carefully considering the properties and applications of nanodiagnostics in clinical imaging.
Theranostic medicine
The parallel development of nanomedicine for therapeutics and diagnostics has led to the development of nanotheranostics, which combines the 2 within a single formulation.4
These formulations are utilized for multiple purposes, including monitoring the biodistribution and targeted site accumulation of nanomedicines, visualizing triggered drug release, and assessing therapeutic efficacy over time. Nanotheranostics offer the potential to personalize chemotherapeutic interventions by preselecting patients based on their predicted response to treatment and monitoring treatment efficacy longitudinally.
However, the application of nanotheranostics extends beyond simultaneous diagnosis and treatment. It fundamentally aims at patient preselection for therapy suitability, predicting therapeutic responses, and longitudinal monitoring rather than conventional disease diagnosis. This approach helps in identifying patients who are likely to benefit from nanomedicine-based treatments and those at risk of side effects due to off-target drug localization.
Nanomedicine in Practice
Development Process of Nano Medicinal Products
The development journey from conceptualization to commercialization of nanomedicines shares the same outline as conventional drug development but diverges significantly in execution due to unique advantages and challenges:
- Nanomedicine design
The first development step of a nanomedicine involves conceptualizing the nanoparticle design that can address the target need effectively. The target nanoparticle design will vary based on numerous factors such as the payload, target delivery site… - Screening stage
Post-design, the focus shifts to synthesis and physicochemical characterization of the nanoparticles.
This stage is critical as the physical and chemical properties of nanoparticles (e.g size, surface characteristics, PDI…) significantly influence their interaction with biological systems, distribution, and efficacy. Those nanoparticle characteristics are both influenced by the chemistry used, the API, and the chosen production method.
Typically, during this step tens to hundreds of formulations are tested in small volumes (hundreds of µL) due to high reagent costs. - Preclinical testing
Once the most promising nanomedicine is identified, they undergo rigorous preclinical testing to evaluate their safety, biocompatibility, and therapeutic efficacy both in-vivo and in vitro. These tests are more complex compared to regular drugs due to the unique behaviors of nanoparticles in biological environments, such as their distribution in the body and the potential to bypass biological barriers. - Clinical trial, Scale-Up and Manufacturing:
Once the preclinical tests are completed, the next step of the process involves clinical testing on humans.
In this context, scaling up the production of nanomedicines poses significant challenges, including maintaining product uniformity and stability, which no current formulation method allows in extenso. The manufacturing processes for nanoparticles often require advanced technologies that can control the conditions precisely to avoid batch-to-batch variations. - Regulatory Approval and large scale production: The regulatory pathways for nanomedicines are more stringent and complicated due to the novel properties and potential risks associated with nanoparticles. Regulatory agencies assess not only the therapeutic efficacy and safety but also the environmental impact and potential toxicity of the nanoscale materials.
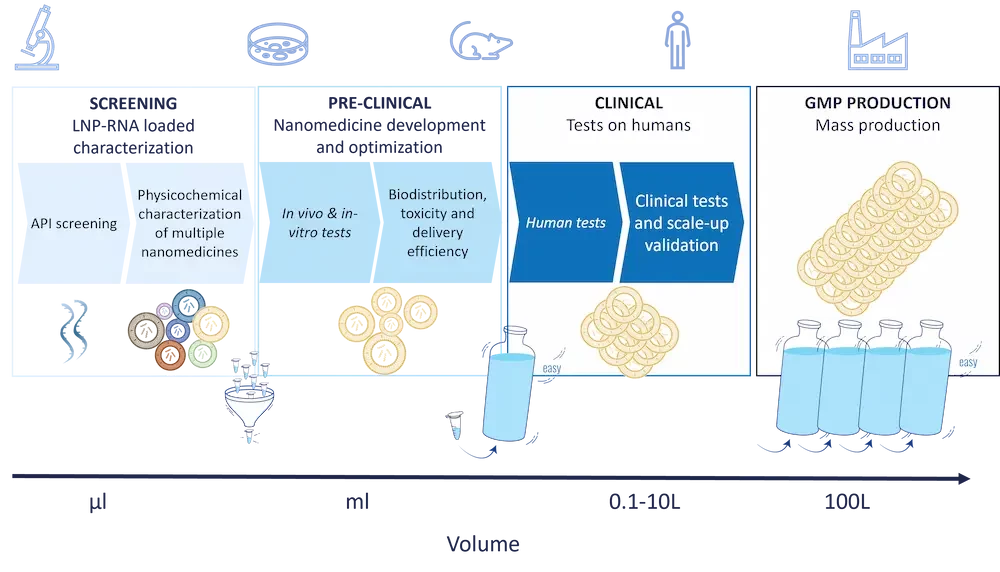
Nanomedicine manufacturing
As described above, the manufacturing process of nanoparticles is a key element to consider for a successful nanomedicine development due to its critical impact on the nanoparticle characteristics, and thus efficiency, and the required scalability.
Numerous methods are available for the synthesis of nanoparticles, and their choice will greatly differ depending on the sought nanoparticle types and characteristics
Organic nanoparticle manufacturing
Manufacturing organic nanoparticles typically involves bottom-up approaches like self-assembly in a solvent. Here, lipids or polymers are dissolved into a solvent, before being mixed with an anti-solvent – generally water – where they precipitate to form nanoparticles. Common methods include bulk mixing or thin-film evaporation, which initially produce a broad nanoparticle distribution. This distribution is then refined using high-energy methods like sonication or high-pressure homogenization. For enhanced precision and repeatability, developers are increasingly turning to microfluidics-based methods, which allow for finer control over mixing conditions and resultant nanoparticle characteristics. More can be found in our LNP manufacturing review.
Inorganic nanoparticle manufacturing
When it comes to inorganic nanoparticles, considering the variety of nanoparticles that can be made, an even broader range of techniques can be used. They include bottom-up approaches such as chemical reduction, sol-gel processes, electrochemical synthesis, and even solvent-based methods – the same as the one used in organic nanoparticle manufacturing – including the Stober process for instance. Top-down approaches are also utilized, where larger material pieces are progressively ground down to the desired nanoparticle size.
Challenges and Future Prospects in Nanomedicine
Current Challenges Facing the Field
Nanomedicine offers transformative potential in healthcare, yet it faces several significant hurdles that must be addressed to maximize its impact:
- Safety Concerns: The foremost challenge in nanomedicine revolves around the safety of nanoparticles used in medical applications. Due to their small size and large surface area, nanoparticles can interact with biological systems in unpredictable ways, potentially leading to toxicity issues not seen with larger-scale materials. Ensuring biocompatibility and reducing adverse immune reactions remain critical areas of research.
- Toxicity: Nanoparticles can accumulate in various organs, such as the liver, spleen, and kidneys, leading to potential organ-specific toxicity. Understanding and mitigating the toxicological impacts of nanoparticles is essential for advancing their safe use in medical treatments.
- Technical Barriers: Despite advancements, several technical obstacles impede the broader application of nanomedicine. These include the difficulty of scaling up production without compromising the quality and uniformity of nanoparticles, challenges in storing and handling nanoscale materials, and the complexity of designing nanoparticles that can navigate the body’s immune system and precisely deliver drugs to targeted cells.
- Environmental Impact: The production, use, and disposal of nanoparticles can have unintended environmental consequences. Research is needed to understand the lifecycle impacts of nanoparticles and to develop sustainable practices for their manufacture, use, and disposal.
Future Trends and Directions in Nanomedicine Research and Application
Looking ahead, nanomedicine is poised to undergo rapid growth and evolution, driven by several promising research directions and application trends:
- More Targeted Delivery Through Active Targeting: Future research aims to enhance the specificity of nanoparticle delivery systems through active targeting. This involves functionalizing nanoparticles with ligands or antibodies that bind selectively to disease-specific markers, thereby improving the delivery of therapeutic agents to the intended disease sites while minimizing effects on healthy tissues.
- Integration with Artificial Intelligence (AI): AI and machine learning are increasingly being integrated within nanomedicine development process to optimize the design and functionalization of nanoparticles. AI can help predict nanoparticle behavior in the body, enhance drug delivery systems, and personalize treatments based on patient-specific data.
- Development of Multi-functional Nanoparticles: Researchers are focusing on creating multifunctional nanoparticles that can diagnose, monitor, and treat diseases simultaneously. These theranostic nanoparticles hold the potential to revolutionize personalized medicine, providing real-time monitoring of treatment efficacy and adjusting therapies dynamically.
- Expansion in Gene Therapy: Nanoparticles are becoming critical in the delivery of genetic material for gene editing and therapy. Techniques such as CRISPR/Cas9 are likely to rely increasingly on nanocarriers to effectively and safely deliver gene-editing tools into cells.
- Regenerative Medicine: Nanotechnology is set to play a pivotal role in regenerative medicine by creating scaffolds that mimic tissue structure and function. These nanostructured materials can support the growth and differentiation of stem cells, opening new avenues in tissue engineering and organ regeneration.
Conclusion on nanomedicine
Nanomedicine represents a groundbreaking fusion of nanotechnology and medical science, transforming the approach to diagnostics, therapeutics, and regenerative medicine. With roots stretching back to the mid-20th century, this field has continuously evolved, offering innovative solutions that leverage the unique properties of nanoscale materials. The development of nanomedicine has catalyzed advancements in targeted drug delivery, improved imaging techniques, and provided new platforms for gene therapy and tissue regeneration.
As we look forward, the integration of artificial intelligence promises to further refine the development and application of nanomedicines, enhancing their design and functionalization for more personalized treatment strategies. The ongoing research into multifunctional nanoparticles and active targeting exemplifies the dynamic nature of this field, aiming to produce theranostic tools that not only treat but also monitor and adjust to the therapeutic response in real-time.
With each advancement, nanomedicine continues to push the boundaries of what is possible in healthcare, heralding a future where medicine is not only reactive but predictive, personalized, and precise. This evolution underscores a pivotal shift from conventional medical paradigms to a more integrated and technology-driven approach, setting the stage for significant impacts on global health outcomes.

Looking for the right LNP formulation solution?
Reach out to us to learn how we can help!
References
[1] JACOB F, MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961;3:318-356. doi:10.1016/s0022-2836(61)80072-7
[2] https://doi.org/10.1002/jps.2600620910
[3] https://doi.org/10.1186/2162-3619-1-10
[4] Rizzo LY, Theek B, Storm G, Kiessling F, Lammers T. Recent progress in nanomedicine: therapeutic, diagnostic and theranostic applications. Curr Opin Biotechnol. 2013;24(6):1159-1166. doi:10.1016/j.copbio.2013.02.020