Triple-negative breast cancer therapies : nanomedicines as innovative treatments
Abstract
Triple negative breast cancer (TNBC) remains hard to treat, but breast cancer nanomedicine offer fresh hope. This article surveys both non targeted and targeted nanocarriers including lipid nanoparticles, liposomes, and exosomes that improve drug stability, delivery, and specificity. It also presents emerging strategies such as cancer nanovaccines and inorganic nanoparticles to enhance therapy and radiotherapy personalization.
Breast cancer is one of the main causes of death in women population. While all genders are affected by this cancer, only 1% of diagnosed patients are men. Cardiovascular diseases and cancer are the top mortality causes in women and breast cancer represents 24.5% of all cancer diagnosed, resulting in nearly 700 000 deaths in 2020 for 2 260 000 cases reported. Triple-negative breast cancer (TNBC) shows increased aggressivity and a lower 4-year survival rate (77%) compared to other breast cancer types (82.7 to 92.5%) [2]. To date, breast cancer and TBNC remain an unmet challenge, leaving people and especially women helpless in many situations.
In a recent paper published in Small, Sofia Torres Quintas and colleagues, from Porto University, reviewed the potential of nanomedicines to treat triple-negative breast cancer.
What are triple-negative breast cancers?
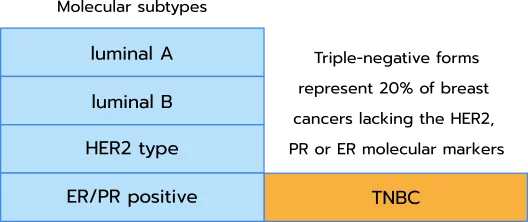
Breast cancers may be categorized according to molecular markers which define their subtypes. Based on these subtypes, four categories can be identified: luminal A, luminal B, human epidermal growth factor receptor 2 (HER2) type, and estrogen (ER)/progesterone receptor (PR) positive. However, approximately 20% of tumors do not present one of these markers and are called “triple-negative”. These tumors are particularly aggressive and do not respond to hormonal therapies. While other strategies are applied (surgery or conventional therapies), these cancers tend to relapse in 40% of cases, leading to metastasis and numerous deaths.
Being negative to molecular markers, these tumors cannot be specifically targeted. Alternative strategies such as nanomedicines appear today as promising solutions to overcome the drawbacks of conventional therapies.
What are nanomedicines?
Several objects fall into the nanomedicine classification. The community agrees on identifying them as spherical nano-objects within 1 to 1000 nanometers diameter wide. Although, a large majority of nanomedicine developed are around 100 nm in size. Their function is to carry an active pharmaceutical ingredient (API) into tissues and cells (specifically or not) explaining why the term “nanocarriers” is often used in the literature. To better understand the different types of nanocarriers, a detailed review is available here.
In their review, Torres Quintas and coworkers [1] provide key features for nanocarriers to be effective:
- Nanocarriers should be physiologically degradable by the body, therefore constituted of components allowing for such.
- Nanocarriers should reduce the undesired toxicity and potential side effects through targeted on-site delivery or reduced dosage in healthy tissues.
- Nanocarriers components should have short half-life and be cleared quickly by the body.
- Nanocarrier must limit the development of immune reaction or resistance development.
The following table outlines formulations which are already approved by drug agencies and used as breast cancer treatments.
| Formulation (API) | Nanocarrier | Year of approval (Location) |
|---|---|---|
| Doxil (doxorubicin) | PEGylated liposome | 1995 (FDA),1996 (EMA) |
| Myocet (doxorubicin) | Non-PEGylated liposome | 2000 (EMA) |
| Abraxane (paclitaxel) | Albumin-bound nanoparticle | 2005 (FDA) |
| Lipusu (paclitaxel) | Liposome | 2006 (China) |
| Genexol-PM (paclitaxel) | Polymeric mycelle | 2007 (South Korea) |
Torres-Quintas et al. outline four types of nanocarriers which are or may be used for breast cancer treatment:
- lipid nanoparticles
- liposomes
- polymeric nanoparticles
- exosomes
The first three classes belong to the lipid-based nanoparticles family while exosomes differ with their endogenous origin.
Which nanomedicines are already approved for TBNC treatment?
In the past decades, the convergence of nanomedicine and genetics led to the development of cancer nanotherapeutics known as cancer vaccines. The strategy consists in encapsulating an API, molecule, peptide, mRNA, or siRNA…, in a nanocarrier to:
- reduce the API’s toxicity
- protect the API from physiological degradation once administrated to the patient,
- improve the API on-site delivery using e.g. targeting approaches
The variety of APIs combined with the different types of nanocarriers leads to a wide range of formulation possibilities, some established and largely used in both the R&D and pharmaceutical markets (mainly Covid-related formulations), others yet to be explored and characterized. The need for solutions to screen these tailored formulations and further scale up their manufacturing remains a challenge where InsideTx technologies bring solutions.
In the following table, we describe some of the encapsulated APIs currently in preclinical-stage formulations aiming at treating breast cancer.
| API | Type | Mechanism of action |
|---|---|---|
| Doxorubicin (DOX) | Antibiotic | Topo-isomerase II inhibition, DNA/RNA polymerase intercalation, free-radicals formation… Exact antitumoral (cytotoxic) activity yet unknow. |
| Paclitaxel (PTX) | Molecule | Antimicrotubular agent. Inhibition of microtubules depolymerization promoting their stability and resulting in mitotic spindle dynamic assembly defaults. |
| Mitomycin C (MMC) | Antibiotic, antineoplastic | Alkylating effect affecting DNA conformation during cell cycle. |
| Cisplatin | Antineoplastic | Similar to alkylating agents: inhibition of DNA linking bridges affecting its conformation during cell cycle. |
| Vinorelbine | Antineoplastic | Inhibition of tubulin polymerization affecting cell division. |
| Decitabine (DAC) | Antineoplastic | Inhibition of DNA methyltransferases resulting in hypomethylation of gene promoters and therefore affecting oncogene expression and/or inducing cell death mechanisms. |
| Panobinostat (PAN) | Antineoplastic | Histone deacetylase inhibition resulting in increased histone acetylation (epigenetic modification) inducing chromatin relaxation and transcription activation. Acetylated histone accumulation induces cell cycle arrest and/or apoptosis. |
| Slug miRNA | miRNA | Inhibition of SLUG protein translation involved in the regulation of cell migration. |
| Surviving siRNA | siRNA | Inhibition of surviving protein translation involved in cell division regulation and apoptosis inhibition. |
| LDHA siRNA | siRNA | Inhibition of lactate dehydrogenase protein translation, an enzyme catalyzing the conversion of pyruvate to lactate under anaerobic conditions, key in the altered glycolytic metabolism that is a feature of cancer cells |
Doxorubicin is an antibiotic commonly used as a chemotherapy for several cancers including stomach, ovarian and breast cancers. It has been approved as a nanomedicine back un 1995 and 1996 respectively by the Food Drug Agency and by the European Medical Agency. This formulation was a breakthrough advance in cancer therapy as the nanomedicine was addressing the key features mentioned earlier in this review: (i) as the first nanomedicine using PEGylated lipids, doxorubicin was better protected from degradation; (ii) improved payload and stability of the drug; (iii) side effects were reduced, particularly cardiac toxicity, therefore bringing better patient compliance and quality of life during the therapy.
The second molecule, paclitaxel is a mitotic disrupter affecting microtubule depolymerization and other key tubulin dynamics resulting in a global antimicrotubular activity and consequent cell division arrest. Paclitaxel (PTX) has been first approved by the FDA as a nanoparticle formulation and as a liposome and a polymeric nanoparticle in China and South Korea respectively.
Clinical-stage nanomedicines for triple-negative breast cancer therapies
These ongoing clinical tests can be divided into two strategies: non-targeting and targeting nanocarriers. One major advantage of nanomedicines relies on their ability to be functionalized using additional agents, chemically bound to the nanocarrier, to specifically address cells or tissues.
On the first hand, nontargeting nanomedicine strategies cannot, therefore, target tumors. Nevertheless, candidates are currently under clinical validation as they still bring advantages over conventional therapies (payload, stability, lower dosage, better degradation, improved tolerance…).
- A nanostructured lipid-carrier (SLN, a mix of liquid and solid lipids) encapsulating doxorubicin and tocopherol succinate, the two compounds showing synergistic effects leading to improved survival together with lower toxicity in mice [3].
- A modified niclosamide, SLN-encapsulated, showing tumor regression and increased survival in mice [4].
On the second hand, one can functionalize the nanoparticle to specifically target a cell type or a cell component. So-called targeted strategies are prone to be more selective and consequently, less toxic as they do not target healthy tissues.
In a study, Zhang and colleagues functionalized SLNs with cyclic RGD peptide targeting αvβ3 integrin, a protein overexpressed in TNBC tumors. The encapsulated doxorubicin and mitomycin C could accumulate specifically in tumoral cells leading to targeted cytotoxicity. In vivo, results in xenograft mice showed encouraging results [5].
A second example, also aiming at reducing cancer drug resistance, is provided by Guo et al. in 2019 [6]. In their work, authors developed a dual complementary liposome, encapsulating doxorubicin, carrying linked antibodies directed against the proteins intercellular adhesion molecule-1 (ICAM1) and epithelial growth factor receptor (EGFR). Results showed better binding and internalization of the liposomes and, as expected, decreased receptor signaling, an encouraging result to limit tumoral resistance.
In the following table, a list of commonly used APIs against TNBC is provided. Although still in the preclinical steps, these candidates bring promising results for future triple-negative breast cancer nanotreatments.
| Formulation | API | Target | Advances | Refs. |
|---|---|---|---|---|
| RGD peptide-functionalized lipid-polymer hybrid NPs (RGD-SLN) | doxorubicin, mitomycin C | αvβ3 integrin | Reduction of lung metastasis; decrease of liver and heart toxicity; increase of the median survival time | Zhang et al., Acta Pharmacol. Sin. 2017 |
| Hyaluronic acid-modified dendrimeric NPs | doxorubicin, cisplatin | CD44 | Inhibition of tumor growth, without noticeable toxic effects | Guo et al. Acta Biomater 2019 |
| Dual complementary antibody-functionalized liposomes | doxorubicin | ICAM1 & EGFR | Decrease in tumor growth | Guo et al., Sci. Adv 2019 |
| Folic acid-functionalized NLCs | Paclitaxel | Folic acid receptor | Antitumor effects without side effects; reduction of tumor burden | Zhang et al., Int. J. Pharm., 2019 |
| Dual targetting Glu6/FA functionalized liposomes | Paclitaxel | uPA receptor | Inhibition of MDA-MB-231 cells growth; accumulation of nanoformulation in the bones | Yang et al., Chem. Phys. Lipids 2020 |
| PEGylated peptide modified liposomes | vinorelbine, slug miRNA | NPR | Silencing of Slug expression; inhibition of TGF-β1/Smad pathway; enhancement of antitumoral activity | Yan etal., Int. J. Nanomed. 2019 |
| Functionalized lipid nano emulsions (LNEs) | decitabine, Panobinostat | LPC receptor, LPA receptor | Reduction of cancer cells viability | Ruoslahti, Adv. Drug Delivery Rev. 2017 |
| Polypeptide modified liposomes | surviving siRNA | HSP gp96 | Inhibition of breast cancer xenograft growth; inhibition of surviving expression in tumors | Liang et al., Mater. Sci. Eng. 2021 |
| Vesicular cationic lipid-assisted NPs | LDHA siRNA | LDHAC | Neutralization of tumor pH; increase of CD8+ T and natural killer cells infiltration; reduction of tumor burden | Zhang et al., Nano Lett. 2019 |
Cancer nanovaccines : novel nanomedicines merging immunotherapies and nanoformulations
Triple-negative breast cancers may benefit from the convergence of immunotherapeutic strategies and nanocarriers.
Many tumors, so-called cold tumors, escape from being addressed by the patient’s immune system. Engineering the cancer cells so that the immune system can recognize tumor antigens and eliminate them. However, delivering the immunotherapeutic molecules remain a challenge as they need to interact with several cell type to induce antitumor immune response. This where scientists started to use NPs as cancer vaccines nanocarriers. Several strategies have been tried leading to promising preclinical results. These formulations usually combine a known antitumoral API such as those previously listed (doxorubicin, paclitaxel) and an immune system promoting molecule.
| Formulation | API | Target | Advances | Refs. |
|---|---|---|---|---|
| Detachable immune-liposomes (Lipids) | paclitaxel | CD47 | Increased Lipids tumor accumulation; reduction of tumor growth; suppression of lung metastasis | Chen et al., Nano Lett. 2021 |
| Dual-functional albumine nanoparticles | paclitaxel, PI3Kγ inhibitor | PI3Kγ | Reduction of tumor growth and metastasis formation; improvement of mice survival | Song et al., Sci. Transl. Med. 2022 |
| Dual-functional polymeric micelles | doxorubicin, indoleamine inhibitor | indoleamine | Apoptosis of 4T1 cells | Lan et al., Appl. Mater. Interfaces 2020 |
| Dual-functional polymeric micelles | paclitaxel, IL-12 | IL-12 receptor | Reduction of tumor growth | Lu et al., Adv. Healthcare Mater 2020 |
| Dual-functional PEGylated liposomes | doxorubicin, IL-12 | IL-12 receptor | NV-DOXIL-2 accumulation within the tumor; suppression of tumor growth | Wu et al., Nanomedicine 2019 |
| Dual-functional nanosatellites | doxorubicin, resveratrol | Transferrin receptor 1, C-type lectin receptors | Induction of ICD in 4T1 cells; repolarization of M2- into M1- macrophages | Du et al., Biomacromolecules 2019 |
| Functionalized cGAMP-NPs | cGAMP | STING receptor | Reprogramming of tumor microenvironment; inhibition of tumor growth; increase mice survival | Cheng et al., JCI Insight 2018 |
| Functionalized immuno-NPs | c-di-GMP, monophosphoryl-lipid A | STING and TLR4 receptors | Decrease of tumor growth; increase of APCs and NK cells in the blood and at the tumor | Atukorale et al., Cancer Res. 2019 |
| Polymeric nanoparticles | granzyme B | Cell-penetrating peptide TAT and CD44 | Tumor growth suppression by release of granzyme B to tumors | Qian et al., Theranostics 2019 |
| Lipid nanoparticles | plasmid encoding a protein to trap IL-10, CXCL12 | IL-10 and CXCL12 | Reduction of tumor growth; prolongation of host survival | Shen et al., ACS Nano 2018 |
| Chitosan polymeric nanoparticles | Chitosan, poly (γ-glutamic acid) | NA | Reprogramming of immature DCs and immunosuppressive macrophages toward an immunostimulatory profile in vitro; impairing cancer cell invasion | Castro et al., Mater. Today Adv. 2022 |
| Nano-emulsion | Puerarin | ROS | Improve the solubility and bioavailability of puerarin; reduction in the expression of TAFs; reduction of tumor growth | Xu et al., Biomaterials 2020 |
Torres-Quintas and co-authors follow-on by reviewing other types of nanomedicines, namely enhancers of radiotherapies and exosomes.
These nanocarriers differ from the organic-based nanoparticles described and used so far in this review.
Indeed, radiotherapy strategies use inorganic nanoparticles composed of high atomic numbers such as silver, gold, gadolinium and iodine. The objective is to enhance the efficiency of radiotherapies or limit the radiation to the tumoral cells. TO this extend, major improvements have been performed in enhancing RT responses against TNBC in preclinical studies.
Exosomes are extracellular vesicles, a totally different class of nanocarriers, as they are not manufactured through lab techniques but are from endocytic origin. One major features of exosomes is that they naturally load DNA, mRNA, LnRNA, siRNA, proteins…which improve their efficacy notably through intercellular communication and ease of cellular penetration. Similarly to NPs, exosomes can be functionalized and/or used in combination with immunotherapies. As a consequence, some exosome-based cancer vaccines hare under preclinical studies for TNBC treatment.
Conclusions & perspectives on nanomedicines for TBNC treatment
In this review, the authors nicely review a large set of nanoparticle formulations and exosome nanocarriers to fight against triple-negative breast cancer. Hybrid approaches mixing nanoparticles, liposomes, exosomes, or exosomes/cell membranes are an additional illustration of the wide set of ingredients available to develop efficient TNBC nanomedicines. Yet, these nanocarriers require deeper characterization and studies.
The rise of cancer vaccine strategies mixing nanocarriers and immunotherapies showed promising anti-tumoral effects on several in vitro and in vivo models. Some of these strategies are to date (as of April 2023), in clinical trials. NCT04249167, an albumin nanoparticle encapsulating Atezolizumab (an anti-PD-L1 monoclonal antibody) and paclitaxel is under phase I. NCT02530489 is a similar strategy in phase II. Close to these formulations, NCT03971409 encapsulates doxorubicin with avelumab (an anti-PD-L1 monoclonal antibiotic) and binimetinib (a MEK1-2 inhibitor). This phase IV study is close to the phase I NCT05029999 combining doxoribiscin and CD40 agonist, Flt3 ligand.

Looking for the right LNP formulation solution?
Reach out to us to learn how we can help!
References
[1] Torres Quintas S, Canha-Borges A, Oliveira MJ, Sarmento B, Castro F. Special Issue: Nanotherapeutics in Women’s Health Emerging Nanotechnologies for Triple-Negative Breast Cancer Treatment. Small (Weinheim an der Bergstrasse, Germany). 2023 Mar:e2300666. DOI: 10.1002/smll.202300666. PMID: 36978237.
[2] Wu Q, Siddharth S, Sharma D. Triple Negative Breast Cancer: A Mountain Yet to Be Scaled Despite the Triumphs. Cancers (Basel). 2021 Jul 23;13(15):3697. doi: 10.3390/cancers13153697. PMID: 34359598; PMCID: PMC8345029.
[3] B. Lages, R. S. Fernandes, J. O. Silva, A. M. de Souza, G. D. Cassali, A. L. B. de Barros, L. A. Miranda Ferreira, Biomed. Pharmacother. 2020, 132, 110876.
[4] K. Pindiprolu, P. T. Krishnamurthy, V. R. Ghanta, P. K. Chintamaneni, Nanomedicine 2020, 15, 1551.
[5] Zhang, P. Prasad, P. Cai, C. He, D. Shan, A. M. Rauth, X. Y. Wu, Acta Pharmacol. Sin. 2017, 38, 835.
[6] Guo, J. Yang, D. Liu, L. Huang, G. Fell, J. Huang, M. A. Moses, D. T. Auguste, Sci. Adv. 2019, 5, 5010