Lipid-based nanoparticles (LBNPs) are advanced delivery systems designed to transport therapeutic molecules with high precision and stability. This guide explains their structure, formulation methods, and role in improving drug bioavailability, enabling targeted treatments in gene therapy, vaccines, and other innovative biomedical applications.
Over the past three decades, the development of lipid-based nanoparticles (LBNPs) technologies as a carrier for drug delivery was key to the successful development of numerous cancer treatments (Doxil, 1995) and vaccines, including the mRNA-based COVID-19 vaccine, which allowed unprecedented achievement at a time of urgent medical need.
The high versatility, stability, and biocompatible nature of these nanomedicines have permitted the delivery of a variety of bioactive materials to a specific location of the body while protecting them from enzymatic degradation during the delivery process. Thanks to their biodegradable nature, lipid-based nanoparticles are currently widely used in the cosmetic industry and the drug delivery fields as a carrier to encapsulate, deliver, and release therapeutics to a specific location in the body.
The following article aims at describing the currently available lipid-based nanoparticle technologies, their synthesis process, and their main applications.
History of the lipid-based nanoparticles
Lipid-based nanoparticles have their origins in the 1960s when scientists first discovered liposomes and quickly identified them as a possible solution for active pharmaceutical ingredients (APIs) with low water solubility. However, it took more than 30 years and numerous improvements for the first liposomal drug product, Doxil (doxorubicin hydrochloride liposome injection) for the treatment of breast cancer to be approved by the FDA in 1995.
The term lipid-based nanoparticles came into use in the early 1990s with the emergence of nanoscience and nanotechnologies. In the following years, several other types of lipid-based nanoparticles such as Lipid nanoparticles (LNPs), Solid lipid nanoparticles (SLNs), and Nanostructured lipid carriers (NLCs) were developed and widely used thanks to their improved drug-loading capability and stability.
One can distinguish 3 main generations of lipid-based nanoparticles:
- The first generation, referred to as conventional liposomes or “pure lipid approach”.
- The 2nd generation involved the use of PEGylated lipids to functionalize the nanoparticle surface. Indeed, coating of the nanoparticle surface by a neutral polymer, such as PEG, helps escape macrophage uptake thanks to steric diffusion thus increasing circulation lifetime. A longer lifetime thus translates into an improved EPR (Enhanced Permeability and Retention) effect, leading to nanoparticle accumulation into tumor tissues and/or liver.
- The 3rd generation of lipid-based nanoparticles involves active and passive targeting of the nanoparticle surface by numerous different methods, such as illustrated below.
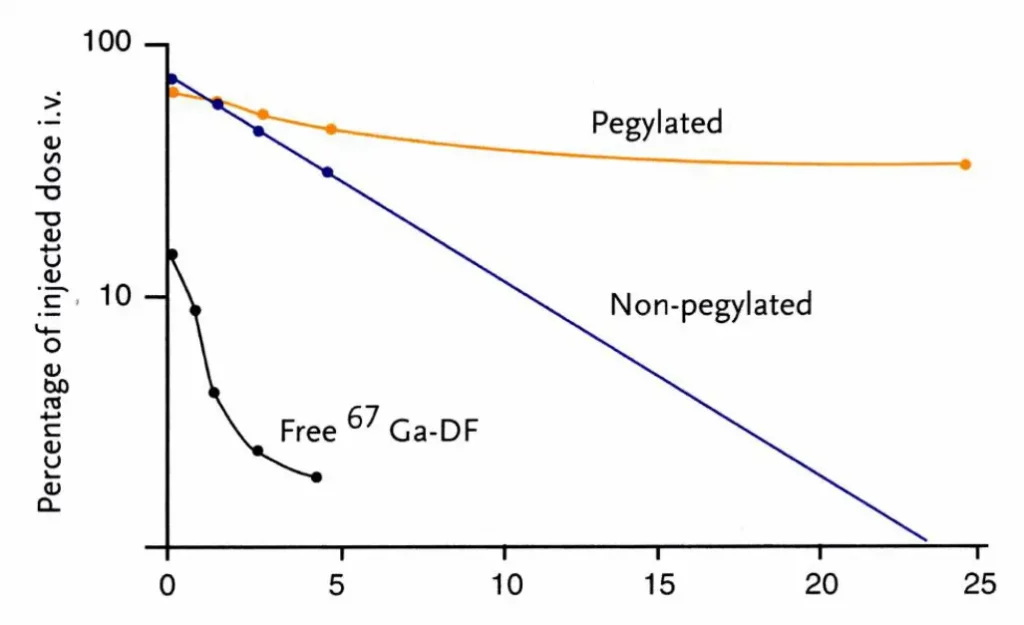
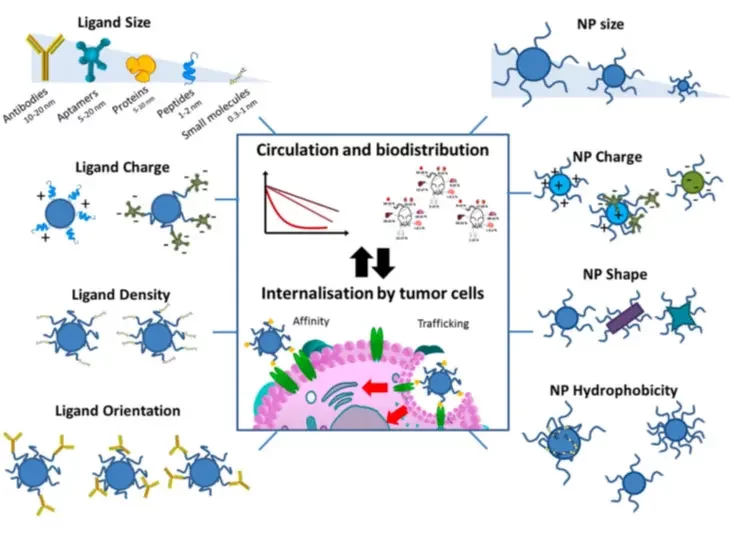
In recent years, LNBPs have become an increasingly popular method of drug delivery. They have been used to deliver a wide range of molecules, including proteins, peptides, nucleic acids, and small molecules.
Lipid-based nanoparticles came in broad light to the public in 2020 during the Cov-Sars19 pandemic, as they were a core component of the latest generation of mRNA vaccines from Pfizer-BioNTech (Comirnaty) and Moderna (Spikevax)
However, there remain some limitations to the existing lipid-based nanocarriers, such as difficulties in scaling up their production, and the potential toxicity. The current research challenges on LNBPs are to make them an even more effective, targeted, and safer method of drug delivery.
LBNPs key definitions
Lipid-based nanoparticles
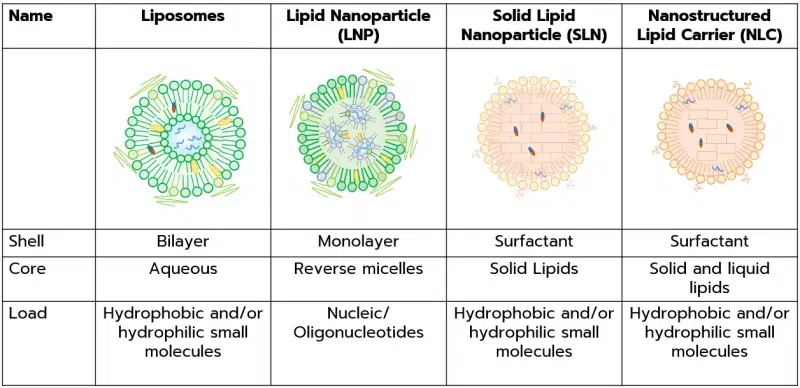
Lipid-based nanoparticles (LBNPs) – or lipid-based nanocarriers – is a general term describing a spherical nanoparticle with an average diameter between 10 and 1000 nm that uses lipid as the main structural component. Lipid-based nanoparticles can be categorized depending on their structure and conformity. The most common ones are liposomes, Lipid nanoparticles (LNP), Solid lipid nanoparticles (SLNs), Nanostructured lipid carriers (NLCs), and Lipid drug conjugates (LDC)
Lipid nanoparticles (LNPs)
Lipid nanoparticle is a controversial term that is sometimes used to describe the whole category of lipid-based nanoparticles or the game changing Nucleic Acid-Loaded Lipid Nanoparticles. We will use the latter definition throughout this article.
In this context, LNPs consist of a lipid shell containing a core composed of reverse micelles encapsulating oligonucleotides such as mRNA, siRNA…
LNPs are generally composed of an ionizable lipid (positively charged at low pH, and neutral at physiological pH), a PEG lipid, a phospholipid, a cholesterol lipid, and the oligonucleotide to be loaded. Find more about them in our review on Lipid nanoparticles (LNP)
Liposomes
First discovered lipid-based nanoparticles, they consist of spherical, closed vesicles in the nano et micrometer size range formed by at least one concentric lipidic bilayer, trapping one or several aqueous vesicles. Liposomes are generally classified by size and lamellarity, the most common ones are:
- Small unilamellar vesicle (SUV): One single lipid bilayer forming one single vesicle typically in the 15 to 30 nm range.
- Large unilamellar vesicle (LUV): One single lipid bilayer forming one single vesicle typically ranging from 100 to 200 nm
- Giant unilamellar Vesicle (GUV): One single lipid bilayer forming one single vesicle larger than 1µm
- Multilamellar vesicle (MLV): Several lamellar phase lipid bilayers with several vesicles
Liposomes can be extremely useful for the delivery of certain drugs, and in mimicking cell functions to study biological systems, such as membrane interaction.
- Solid Lipid Nanoparticles (SLN): 2nd generation of lipid-based nanocarriers, SLNs are mainly composed of solid lipids (such as fatty acids, and fatty alcohols…) and a stabilizing agent (such as a surfactant) which combine to form a surfactant shell surrounding a core matrix of solid lipids.
SLNs are preferred to liposomes as they have no biotoxicity, are highly stable, offer better drug release and targeting control, and can encapsulate both lipo- and hydrophilic APIs.
Nonetheless, the crystalline property of the SLN limits their drug load capabilities. In fact, the possible crystallization process occurring in the nanoparticle can lead to drug expulsion from the formulation during storage. - Nanostructured Lipid Carriers (NLC): NLC is the 3rd generation of solid nanoparticles that appeared in the late 1990s to overcome the main limitations of the SLNs.
Resembling a nanoemulsion, it consists of an SLN modified by incorporating a liquid (amorphous) lipid into the solid core. This permits the formation of nanoparticles with smaller sizes (1 – 100nm) while offering extended drug loading capacity, and better stability upon storage while keeping similar low toxicity. - Lipid Drug conjugates (LSCs) are the newest generation of lipid-based nanoparticles.
LCSs aim at improving the encapsulation capabilities of hydrophilic s compared to NLC and SLNs – which show poor hydrophilic encapsulation efficiency
Lipid-based nanoparticles formulation
Lipids are the main components of the formulation and play a key role in regulating the type of synthesized nanoparticle and its stability, encapsulation, and release of the therapeutics or API. Therefore, lipid selection is key to a successful formulation, being very specific, able to encapsulate with high efficiency, and biocompatible.
LBNP’s typical compositions are the following:
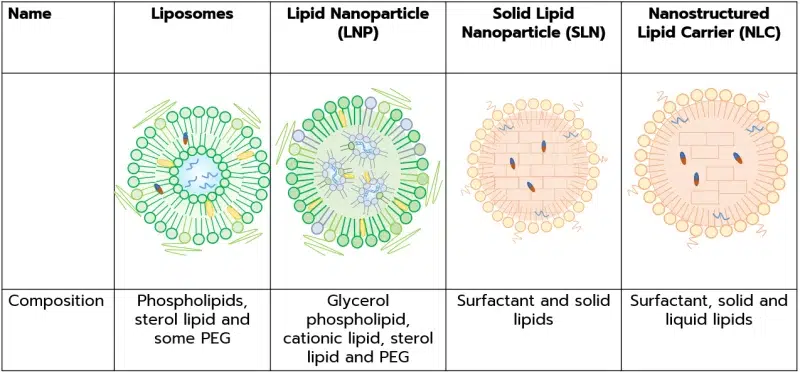
- Liposomes are comprised of phospholipids, especially phosphatidylcholine, and cholesterol, which show an amphiphilic behavior with a hydrophobic tail and a hydrophilic head and a few% of PEG.
The underlying formation mechanism of liposomes is hydrophilic-hydrophobic interactions between lipid/lipids and lipid/water molecules, where to achieve thermodynamic equilibrium, the system will self-assemble to form a bilayer vesicle.
Source: A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents - LNPs are primarily composed of a glycerophospholipid, a cationic lipid, a sterol lipid, and a PEGylated lipid that entrap oligonucleotides in an aqueous phase.
- Ionizable lipid: Core component of the LNP (roughly 50 mol%), the ionizable lipid facilitates nucleic acid encapsulation, its release in the cytosol, as well as reduces the LBNP toxicity.
These unique features are made possible by the Ionizable lipid’s unique ability to change in charge depending on the pH of the lipid. - Glycerophospholipids (typically DSPC): The 1st helper lipid (10 mol%) is the core component of the external LNP membrane.
- Sterol lipid (Typ. Cholesterol): The 2nd helper lipid represents roughly 40 mol% of the SLN and helps the structure to reach a liquid order phase and thus greatly helps with the SLN stability and serves to reduce drug leakage.
- PEG-lipid: smaller molar percentage of lipid components, PEG-lipids have a huge impact on the LNP size, dispersity, aggregation prevention, and stability… and are a key element to the LNP self-assembly via the hydrophilic steric barrier they form at the LNP surface.
- Ionizable lipid: Core component of the LNP (roughly 50 mol%), the ionizable lipid facilitates nucleic acid encapsulation, its release in the cytosol, as well as reduces the LBNP toxicity.
- SLNs and LNCs composition are relatively similar and greatly depend on the type of LBNPs synthesized, yet the general structure is as follows:
- Lipid: Primary components of an NLC, the lipid drives the loading capacity and stability of the formulation.
NLCs are generally made of a mix of a liquid and a solid lipid at a ratio ranging from 70/30 to 99/1, while SLNs are made of 100% of solid lipids
A wide range of solids lipids can be used, nevertheless, biodegradable and non-toxic ones should be preferred. - Surfactant: Highly influencing the particle stability, toxicity and crystallization, the surfactant should duly be chosen depending on the lipid contents of the NLC.
- Lipid: Primary components of an NLC, the lipid drives the loading capacity and stability of the formulation.
Lipid-based nanoparticles synthesis
LNBPs are manufactured using a wide variety of bottom-up processes. The choice of technic will depend on the sought nano carrier properties, and route of administration. They can be classified into 3 different methods:
High-energy methods:
Using equipment able to generate high shear stress forces to break the surface tension and Laplace pressure of the particle, and decreased its size.
The most common methods are high-pressure homogenization, ultrasonic homogenization, membrane extrusion, or supercritical fluid-based methods.
The main limitations of those methods both relate to the high pressure involved that can degrade the lipid and API properties, as well as the limitation of working with small batches.
Some of them also have issues related to the high polydispersities of the synthesized LNPs, and poor batch-to-batch reproducibility.
Low energy-based methods:
Particle reduction is here achieved not using high energy, but either chemical or hydrodynamics mechanisms of the system. Generally based around emulsions (single or double) and temperature-controlled methods, those methods offer good results but are not available for every nanoparticle types. On top of this, they are very operator dependent and unstable in time.
Organic solvent methods:
These method based around self-assembly are currently considered state of the art thanks to the excellent size control, PDI, and encapsulation efficiency they provide.
Generally, the lipids are dissolved in a water-miscible organic solvent (most often ethanol) before being mixed with an aqueous solution containing the API.
These methods rely on rapid diffusion of the solvent in the aqueous phase, which will lead to a drop of solvent concentration in the solution and trigger the self-assembly of the LNP in the solution.
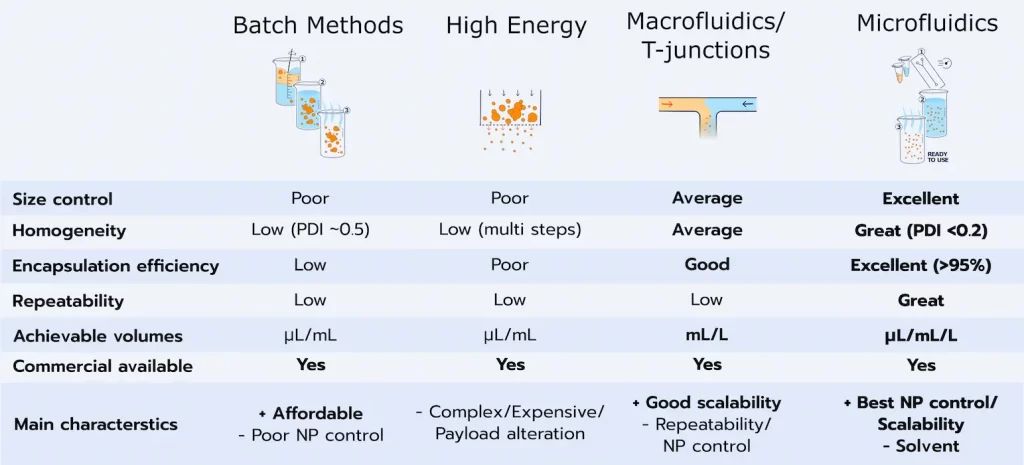
As physical properties of the LNP are based on local mixing rate, large-scale batch methods such as thin film evaporation or large-scale mixing generally do not provide homogeneous lipid-based nanoparticles populations.
To overcome this limitation, microfluidics mixing, which mixes with better control and repeatability thanks to the laminar flow conditions, is currently the most popular method used to overcome those limitations and produce more consistent results.
Lipid-based nanoparticles characterization
As they are mostly used in the pharmaceutical industry, quality control of the Lipid based nanocarriers is critical and requires adequate characterization.
Even though no clear framework has been defined, the “Liposome guidance from Industry” from the FDA (U.S. Department of Health and Human Services Food and Drug Administration & (CDER), 2018), indicates the key parameters that should be used to characterize lipid-based nanoparticles.
- Composition and proportion of each component including API, Lipids, and other ingredients.
- Physicochemical parameters of the LBNP, including:
- Particle size
- Particle size distribution
- Surface characteristic
- Osmolarity
- Net charge/zeta potential
- Morphology and structure (e.g lamellarity…)
- Stability and integrity change
- Parameters of the contained drug such as:
- Encapsulation efficiency (amount of drug contained inside LBNPs vs total amount of drug)
- LNBP drug loading (drug-to-lipid ratio)
- Leakage rate of the drug from the lipid-based nanoparticle
- In-vitro release of the drug substance
More information on the topic can be found in our review on liposomes and LNP characterization guidelines!

Formulate your nanoparticles with our TAMARA Nanoparticle Formulation System
Exemples of lipid-based nanoparticles applications
Current lipid-based nanoparticle applicative landscape
The unique encapsulation and delivery capability of lipid-based nanoparticles have turned them into a real multi-tool for drug delivery.
Since their discovery nearly 50 years ago, numerous LBNP drug formulations have been approved and used, in a wide variety of fields. As of 2021, 21 LBNPs based products encapsulating small molecules have been approved.
Mostly used for cancer treatment, liposomal drugs have also been approved in the fungal, and analgesics field, but also for viral and mRNA delivery, as well as immuno-suppressant…
With the recent success of the mRNA based covid 19 vaccine, numerous clinical trials with LNP-formulated mRNA drugs and vaccines are currently ongoing, both for infections disease ( Rabis, Influenza, Tuberculosis, Covid-19…) also for Cancer immunotherapy, and Protein-replacement therapies.
Drug delivery example: The example of Doxil for cancer treatment
Thanks to their unique biocompatibility and selectivity, LBNP’s largest application field in drug delivery is cancer treatment, as they greatly help increase the effectiveness of anti-tumor agents.
The combined specificity and protection offered by lipid-based nanoparticles permit both to increase the drug resilience in the body and reduce the toxicity of anticancer drugs to regular cells – and thus patient’s side effects.
In 1995, Doxil was the first approved anticancer treatment using lipid-based nanoparticles as a main carrier. Using anthracycline drug doxorubicin as the main active molecule, Doxil helps slow or stop the growth of cancer cells by blocking an enzyme called topo isomerase 2, and is generally used to treat breast or ovarian cancer, as well as multiple myeloma.
To optimize its efficiency, it was decided to entrap the hydrophilic APIs into a liposome bilayer of 100 nm size composed of hydrogenated soy phosphatidylcholine, cholesterol, and DSPE-PEG2000 for the following reasons:
1/ Protect the API and thus prolong the drug circulation time into the body which improved its efficiency
2/ The anthracycline drug doxorubicin, was known to be very cardiotoxic, therefore encapsulating it into a LBNP allowed for a large reduction in cardiac toxicity and improved overall patient well-being.
3/ Encapsulating the API into 100nm liposomes also permits leverage on the EPR (Enhanced permeability and retention) effect, where small molecules in the 100 nm range – such as the drug-loaded liposome- tend to accumulate into tumor tissues.
LNPs for gene therapy delivery, working principle
The most recent and recognized successful use of lipid-based nanoparticles as a drug nanocarrier was during the development of the 2 Covid-19 mRNA vaccines released by Moderna and Pfizer/BioNTech, which were developed, released, and produced at an unmatched speed.
In this context, the role of LNPs was key as it allowed for the delivery the mRNA encoding for the SARS-CoV-2 spike protein into the cytoplasm of the host cells. The mRNA was then translated into the spike protein, which acts as an antigen and thus leads to the development of an immune response for the body. As described in the composition section of this article, the used LNPs contained an ionizable lipid, positively charged at low pH, and neutral at physiological pH.
The LNPs were prepared at low pH, so they can easily form complexes with the negatively charged mRNA. It was then brought back to physiological pH – thus neutral charge – for the drug injection, to minimize the interaction of the lipids with the anionic membrane of blood cells, and thus improve biocompatibility.
Upon entering the endosomes, where the pH is lower than the physiological pH, lipids get ionized, which leads to membrane destabilization and eases the delivery of the mRNA payload.

Want to discuss lipid based nanoparticle fabrication methods more?
Reach out to us to learn how we can help!
References
[1] Bertrand, Nicolas et al. “Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology.” Advanced drug delivery reviews vol. 66 (2014): 2-25. doi:10.1016/j.addr.2013.11.009