
An introduction to novel LNP targeting strategies for optimizing RNA-LNP biodistribution and improving cell specificity
223 views
Read more
Insidetx.com Resources Reviews
This research summary focuses on analyzing how oligonucleotide/ RNA size and the types of lipids used influence the key properties of lipid nanoparticles/RNA-LNP synthesized via microfluidic techniques. The findings suggest that the commonly reported LNP Encapsulation Efficiency (EE%) may not be the most reliable parameter for evaluating formulation performance. Instead, alternative metrics such as EEinput% (Also referred to as Encapsulation Yield) provide a more accurate assessment.
The peer-reviewed article “A careful look at lipid nanoparticle characterization: analysis of benchmark formulations for encapsulation of RNA cargo size gradient” was originally published in Scientific Reports, in 2024 by Gretchen B. Schober, Sandra Story and Dev P. Arya from NUBAD LLC / Clemson University.
Lipid nanoparticles (LNPs) have emerged as effective carriers for therapeutic nucleic acids, with several formulations approved by the FDA. These LNPs are designed to address the challenges of delivering unprotected nucleic acids, such as susceptibility to biological degradation and difficulty in crossing cellular barriers.
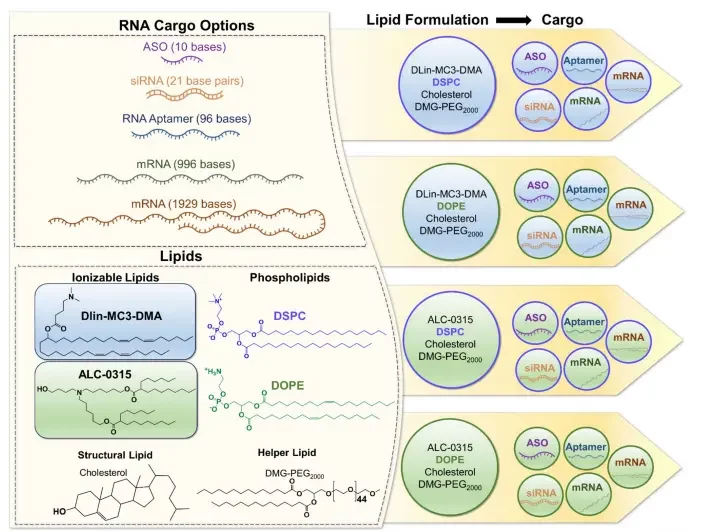
A variety of RNA types are selected for this study, including antisense oligonucleotides (ASO), small interfering RNA (siRNA), RNA aptamers, and messenger RNA (mRNA), reflecting the diverse applications and potential of RNA-based therapies.
Additionally, the composition of LNPs, which is critical for their function, typically involves 4 components: ionizable lipids, helper phospholipids, cholesterol, and polyethylene glycol (PEG)-lipids. The ionizable lipid structure, particularly its impact on RNA encapsulation and delivery, makes it a key area of research and a significant battleground for intellectual property.
In this study, the production of lipid nanoparticles (LNPs) was made using the cutting-edge microfluidic self-assembly process. This method stands out for its ability to produce highly uniform nanoparticles, achieving optimal encapsulation efficiency, precise control and repeatability. Such approach enables a robust correlation between inputs, like cargo size and lipid mix, and outputs, including RNA-LNP size, Polydispersity Index (PDI), Encapsulation Efficiency (EE%), enhancing the effectiveness of the LNPs produced.
Goals of the study:
In this study, LNPs were prepared using microfluidic methods, involving herringbone mixers using a 60:1 lipid to RNA ratio.
4 LNP formulations were evaluated with the same molar ratio of 50:10:38.5:1.5 respectively of ionizable lipid (DLin-MC3-DMA or ALC-0315), phospholipid (DSPC or DOPE), structural lipid (cholesterol) and helper lipid (DMG-PEG2000).
Each of the lipid formulations was tested with 5 different oligonucleotides of different lengths including ASO (10 bases), siRNA( 21 base pairs), aptamer (96 bases), and mRNA strands of different lengths (996 bases and 1929 bases).
RNA-LNP complexes were characterized using standard methods including RiboGreen Encapsulation efficiency measurement, and Size and PDI measurements were carried out using DLS.
The study further explores the encapsulation efficiency (EE) of lipid nanoparticles (LNPs), examining it through two different calculation methods:
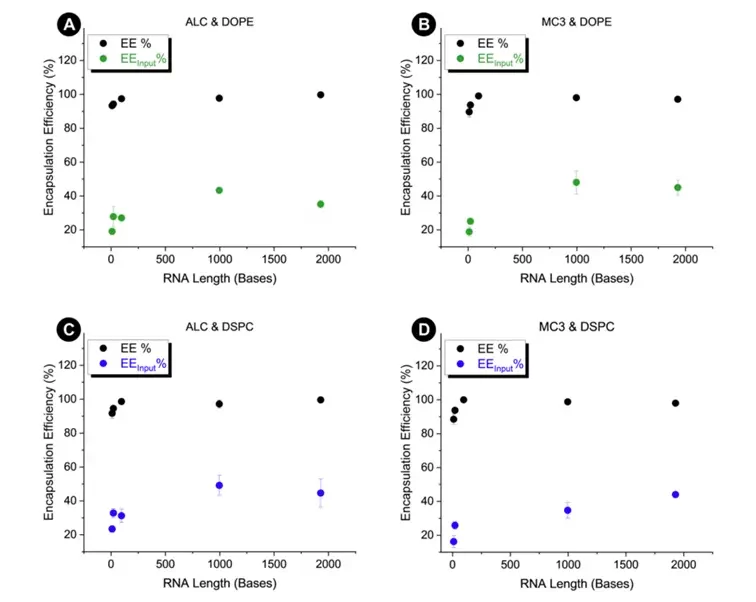
Comparing these 2 approaches, the study shows that actual RNA encapsulation is often lower than reported. This discrepancy suggests that a substantial portion of the input RNA is unaccounted for in the final product, likely due to degradation of the RNA during the process. These degradation mechanisms will artificially increase the EE% by decreasing the unencapsulated RNA concentration.
The paper also points out that larger RNA molecules are encapsulated more effectively in lipid nanoparticles (LNPs) than smaller RNA molecules. This enhanced efficiency is likely due to the stronger electrostatic interactions between the larger RNA and the ionizable lipids in the LNPs. Additionally, the lipid mix also appears to have an impact on the encapsulation efficiency.
These findings underscore the importance of refining LNP formulations, particularly for therapeutic applications, and highlight the importance of accounting for input RNA concentration in encapsulation efficiency evaluations.
Finally, a follow-up study examined the discrepancy between EE% and EEinput% for mRNA with different total lipid concentrations while maintaining a constant RNA-to-ionizable lipid ratio. Results showed that increasing lipid concentration significantly improved EEinput%, highlighting the need to consider input RNA concentration in efficiency calculations. This approach provides more accurate insights into reaction efficiency and aids in optimizing LNP formulations.
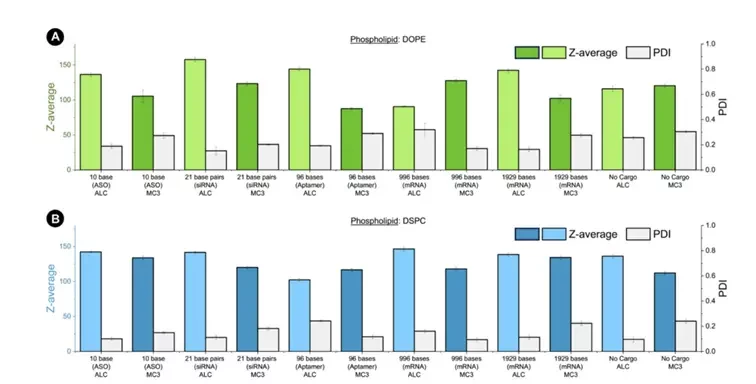
The size analysis showed no clear link between RNA size and LNP diameter in the regime of high lipid excess. This observation is in line with other research which indicates that LNP size depends more on lipid concentration, mixing conditions, and PEG-lipid concentration, rather than the RNA size itself. It was thought that different RNA sizes might affect LNP size due to the varying arrangements of lipids and RNA within the particles, yet it appears not to be the case. However, morphology and internal LNP organization such as packing efficiency might vary and should be further studied.
In summary, the study highlights the critical role of lipid nanoparticles (LNPs) in RNA therapeutic delivery, safeguarding the RNA from degradation. In an analysis exploring the encapsulation efficiency of various RNA cargo sizes, the study unveils an intriguing fact: the traditional methods of calculating encapsulation efficiency often overstate their actual efficiency. It suggests that the concentration of RNA should be factored into efficiency assessments, and alternate methods like EEinput% might offer more accurate encapsulation characterization.
Interestingly, the research indicates that the same formulations yielding positive results for smaller RNA types, like siRNA, may not be as effective for larger RNA types, such as mRNA. This necessitates tailored adjustments in the formulation process depending on the RNA size.
Contrary to expectations, the study finds that the size of LNPs isn’t significantly influenced by the size of the RNA cargo. Instead, the findings point out that the size is largely determined by the specific conditions of the reaction, including the lipid mix, ratio, and microfluidic mixing parameters, rather than the RNA cargo size itself.
In conclusion, this research demonstrates that the LNP formulation plays a more significant role in determining the final characteristics of RNA-LNPs than the RNA cargo itself. Thus, while the formulations should be optimized according to the specific RNA cargo, the overarching influence lies in the formulation conditions themselves.

Reach out to us to learn how we can help!
Schober, G.B., Story, S. & Arya, D.P. A careful look at lipid nanoparticle characterization: analysis of benchmark formulations for encapsulation of RNA cargo size gradient. Sci Rep 14, 2403 (2024). https://doi.org/10.1038/s41598-024-52685-1
Looking to learn more about nanoparticles? Do you need inspiration or a different angle to take on your specific problem? Well, we probably have a Review just for you, feel free to check them out!
See all Nano Reviews
223 views
Read more
230 views
Read more
354 views
Read more
673 views
Read more
3130 views
Read more
862 views
Read more