RNA-LNP morphology is a pivotal quality attribute shaped by lipid composition, formulation conditions, and buffer selection—directly impacting size, PDI, therapeutic efficacy, toxicity, and biodistribution. Optimizing and better understanding RNA-LNP morphology through precise formulation design, advanced characterization is essential for consistent, competitive RNA-drug development success, especially during the scale-up process.
Understanding RNA-LNP Morphology
The development of RNA-lipid nanoparticles (RNA-LNPs) presents a significant challenge due to the intricate interplay between critical process parameters (CPPs)—such as lipid composition, formulation conditions, and buffer selection—and critical quality attributes (CQAs), including nanoparticle size, polydispersity index (PDI), and LNP morphology.
These factors collectively shape the quality target product profile (QTPP), ultimately determining the therapeutic efficacy, toxicity, and biodistribution of the final drug product. As we previously discussed, this challenge is particularly pronounced during scale-up, where modifications to manufacturing processes can substantially impact RNA-LNP characteristics, leading to variability in batch-to-batch consistency.
Among CQAs, parameters like size, PDI and encapsulation efficiency (EE%) are widely used as standard LNP characterization metrics. However, emerging research suggests that these conventional measures alone are insufficient for fully understanding RNA-LNP behavior and ensuring reproducibility.
Additional factors—such as drug loading capacity, the ratio of empty to full nanoparticles, and, critically, LNP morphology—must be considered to achieve a more comprehensive LNP characterization.
LNP Morphology peculiarly plays a pivotal role in the stability, biodistribution, and transfection efficiency of RNA-LNPs, directly influencing their therapeutic potential. Structural variations can impact RNA protection, release kinetics, and cellular uptake, making morphology a key determinant of drug performance. As the field advances, a deeper understanding of LNP organization is crucial for optimizing RNA delivery and designing next-generation nucleic acid medicines.
This review examines recent advancements in RNA-LNP morphology, key LNP formulation parameters affecting structural integrity, and the commercial opportunities that arise from improved morphological LNP characterization. By highlighting the significance of RNA-LNP architecture, we aim to provide insights into strategies for achieving more effective and reproductible therapeutic outcomes.
LNP Morphology definition
RNA-LNP morphology encompasses the structural characteristics of these nanoparticles, including their size, shape, internal organization, and surface properties.
Over the years, our understanding of LNP morphology has significantly evolved. Initially, RNA-LNPs were thought to adopt a simple liposomal structure, as widely assumed in the early 2000s. However, advancements in analytical techniques and structural studies have revealed a far more complex organization, particularly in recent years. By 2021, the perception of LNP morphology had shifted, recognizing diverse architectures that influence stability, encapsulation efficiency, and RNA release dynamics. The schematic below illustrates this progression, highlighting how our interpretation of LNP structure has transformed with scientific advancements.
![Evolution of Understanding LNP Internal Organization & LNP Morphology [1]](https://insidetx.com/wp-content/uploads/2025/08/LNP-Morphology-history.webp)
As of today, and as outlined in the article “A Perspective on Bleb and Empty LNP Structures” by J.B. Simonsen, a diverse array of structural variations can be observed within lipid nanoparticles (LNPs). These include fully loaded LNPs, empty LNPs, LNPs with bleb-like protrusions, multi-compartment LNPs, and hybrid structures combining these different forms.
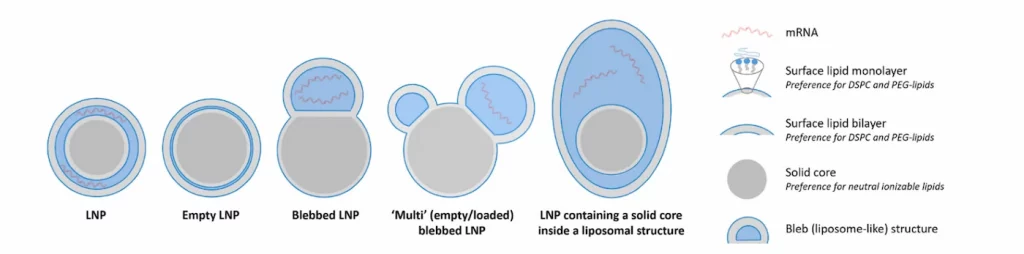
This structural diversity highlights the broad spectrum of accessible LNP architectures, emphasizing that the definition of an LNP is not uniform. Instead, it varies depending on LNP formulation parameters, processing conditions, and analytical perspectives, and composition (RNA, Structural lipid (phospholipid), helper lipid (cholesterol), ionizable lipid and PEG-lipid, each having ) making precise characterization essential for ensuring consistency and therapeutic
How to characterize RNA-LNP morphology ?
While parameters such as size, Polydispersity index (PDI), and surface properties can be readily characterized by using standard techniques like dynamic light scattering (DLS) or nanoparticle tracking analysis (NTA), assessing the shape and internal organization of RNA-LNPs presents a greater challenge. These structural attributes play a crucial role in nanoparticle stability, encapsulation efficiency, and functional performance, yet their characterization requires advanced analytical tools.
To remain competitive in the rapidly evolving field of RNA therapeutics, pharmaceutical and biotech companies must employ state-of-the-art characterization methods. Various techniques are available, each offering a unique balance between data richness, throughput, and accessibility. The table below summarizes key methodologies, highlighting their measurement principles, advantages, and limitations:
| Technique | Measurement Principle | Pros | Cons |
|---|---|---|---|
| Cryogenic Transmission Electron Microscopy (Cryo-TEM) | Direct visualization of LNPs | Provides high-resolution images for detailed morphology and structural characterization | – Time-consuming process – High cost – Susceptible to sample preparation artifacts |
| Small-Angle X-ray Scattering (SAXS) | Scattering of X-rays to analyze nanoscale structures | Offers insight into particle organization and internal architecture | – Indirect measurement – Requires deep expertise for data interpretation |
| Field-Flow Fractionation Multi-Angle Light Scattering (FFF-MALS) | Separation of particles by hydrodynamic size, followed by multi-angle light scattering detection | – Capable of analyzing large sample volumes | – Indirect measurement – Lower level of detail compared to direct imaging – Requires special autosized equipment and expertise |
Each of these techniques has its strengths and limitations, making it essential to select the appropriate method depending on the research question, required throughput, and available resources. Combining multiple approaches often provides the most comprehensive understanding of RNA-LNP morphology, facilitating the development of robust and reproducible RNA delivery systems.
Different LNP Morphologies = Different Biological responses
The morphology of lipid nanoparticles (LNPs) is critical in shaping their biodistribution, pharmacokinetics, and overall therapeutic efficacy. For example, Herrera et al. (Biomaterials Science 9, 4289–4300, 2021) demonstrated that altering the sterol composition in LNP formulations induces distinct morphological profiles and responses in cells.
In their study, they substituted cholesterol with four alternative sterols and characterized the resulting LNP morphologies while evaluating protein expression across three different mRNA doses.
Their findings confirmed that changing the sterol type significantly impacts LNP morphology. Notably, polymorphic-shaped particles—those with non-spherical or irregular forms—led to higher protein expression levels. This improvement is attributed not only to altered nanoparticle structure but also to enhanced endosomal escape, a critical step for efficient mRNA delivery.
Similarly, other studies have shown that elongated or irregularly shaped LNPs accelerate the transition of mRNA from endosomes to the cytoplasm, thereby boosting therapeutic outcomes. Overall, polymorphic particles exhibit improved mRNA release kinetics and more effective intracellular trafficking, resulting in greater transfection efficiency. By tailoring the shape and internal organization of LNPs, researchers can optimize nanoparticle delivery and accumulation in target organs while minimizing off-target effects and toxicity.
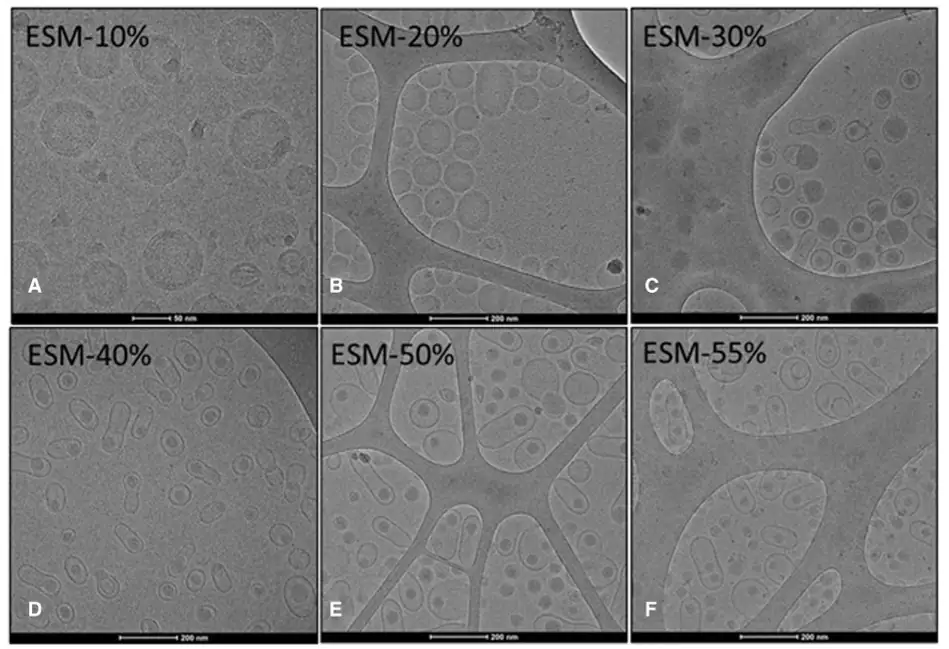
When administered in vivo, these changes resulted in significant differences in protein expression, cellular uptake, and biodistribution. Thus, both the type and the concentration of structural lipids are crucial determinants of LNP morphology, directly impacting potency and targeting.
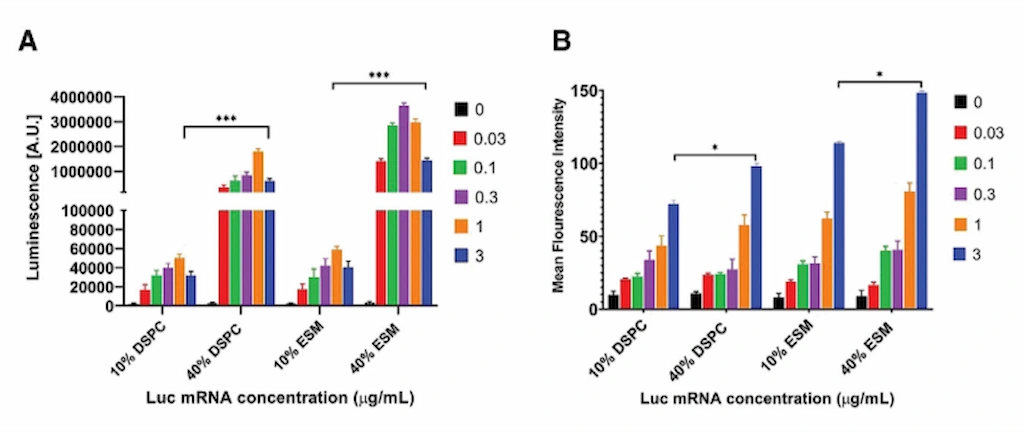
Buffer Composition
Buffer composition, particularly the ionic strength of the aqueous phase, also affects LNP morphology. Increasing the ionic strength has been shown to promote the formation of bleb-like structures within LNPs. These bleb-LNPs are associated with enhanced protein expression, potentially because the bleb structures provide additional protection for the encapsulated mRNA, improving its delivery upon cellular uptake.
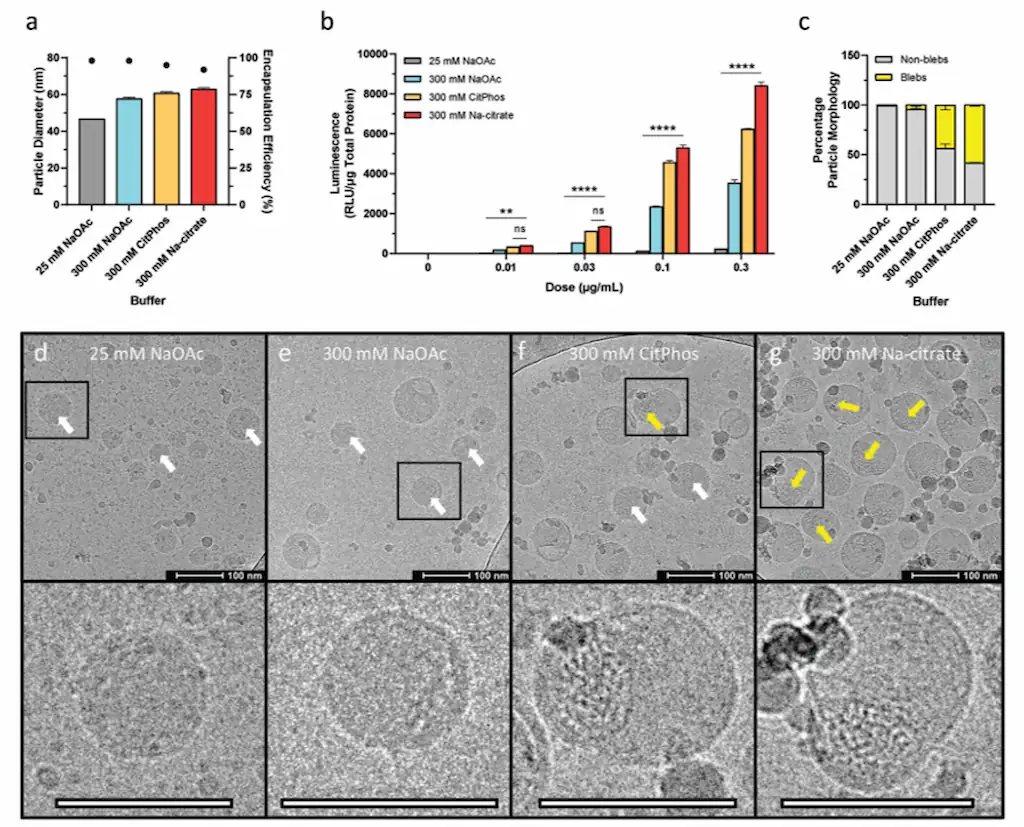
Conclusion:
The Role of RNA-LNP Morphology in Next-Generation Therapeutics
Given the number of factors influencing the LNP internal organization, there is no single, unified model of their structure. Proposed models range from simple multilamellar vesicles to inverted micelle cores and more intricate hybrid architectures.
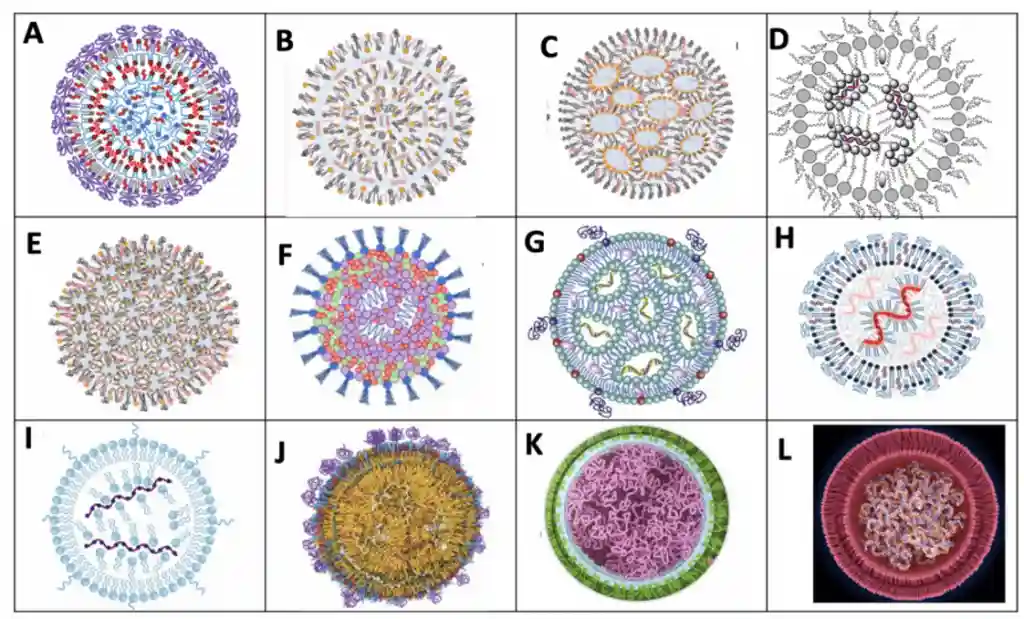
RNA-LNP morphology is not just an academic concept—it is a fundamental driver of therapeutic success as it drives most of the drug characteristics. Companies that integrate advanced LNPs characterization methods, precise LNP formulation strategies, and scalable manufacturing processes will be at the forefront of RNA medicine. Investing in RNA-LNP morphology research and optimization is critical for maintaining a competitive advantage in the rapidly evolving landscape of nucleic acid therapeutics.

Looking to optimize your LNP formulation?
Reach out to us to learn how we can help!
References
[1] Bader, J., et al., Extracellular vesicles versus lipid nanoparticles for the delivery of nucleic acids (2024). https://doi.org/10.1016/j.addr.2024.115461
[2] Simonsen, J. B. A perspective on bleb and empty LNP structures. Journal of Controlled Release 373, 952–961 (2024). https://doi.org/10.1016/j.jconrel.2024.07.046
[3] Chander, N., et al., Lipid nanoparticle mRNA systems containing high levels of sphingomyelin engender higher protein expression in hepatic and extra-hepatic tissues. Mol. Th. Meth. and Clin. Dev. (2023). https://doi.org/10.1016/j.omtm.2023.06.005
[4] Cheng, M. H. Y. et al. Induction of Bleb Structures in Lipid Nanoparticle Formulations of mRNA Leads to Improved Transfection Potency. Advanced Materials 35 (2023). https://doi.org/10.1002/adma.202303370
[5] Szebeni, J., et al., Insights into the Structure of Comirnaty Covid-19 Vaccine: A Theory on Soft, Partially Bilayer-Covered Nanoparticles with Hydrogen Bond-Stabilized mRNA–Lipid Complexes (2023). https://pubs.acs.org/doi/10.1021/acsnano.2c11904