Abstract
Understanding LNP biodistribution is vital for the success and safety of RNA-based therapies. This review examines how lipid nanoparticle properties—such as size, lipid composition, formulation method, and administration route—govern their in vivo distribution. It explores which tissues and organs LNPs commonly reach and discusses strategies to enhance delivery precision, offering mechanistic insights for optimizing biodistribution in therapeutic applications.
Lipid nanoparticles (LNPs) have revolutionized the biotechnology and medicine field by offering a powerful way to encapsulate ribonucleic acids (RNAs). These tiny particles are crucial for delivering synthetic RNAs like mRNA and siRNA into cells, which is key for a wide range of gene and cell therapies, from vaccines and gene editing to cancer treatments. The success of RNA-based therapies, such as the COVID-19 vaccines, depends on LNPs’ ability to protect RNA molecules and ensure they reach their target cells intact.
To make the most of LNPs in treatments, it’s essential to understand their biodistribution – how they spread throughout the body after administration. This distribution affects how effective and safe they are. Several factors play a role in this process, including the size and lipid composition of the LNPs, how they are made, and how they are administered.
This review will explore these factors in detail, aiming to give an insight on the mechanisms behind LNP biodistribution. We’ll explore which organs and tissues LNPs typically reach and how to enhance their delivery precision. By examining the latest research and studies, we hope to provide a clear picture of LNP behavior and how to optimize them for various therapeutic goals. Understanding these aspects is crucial for advancing RNA-based therapies and realizing their full potential in transforming medical treatments.
Introduction on RNA-LNP
How LNPs came to be the gold standard for encapsulating and delivering a unique type of biomolecule : RNAs
Ribo-Nucleic Acids (RNA) are biomolecules that have many different iterations in the biological system (siRNA, mRNA, qRNA…), all of them with specific purposes and mechanisms. mRNA, for example, is a type of biomolecule involved in the production of proteins through their translation in the cell’s cytosol. Being able to use the specific properties of RNA opens the door to many different innovative applications in the treatment and/or prevention of several diseases. For example, it is possible to replace defective proteins silence certain genes (SiRNA, CRISPR) or create “Neo-Antigens” (SARS COV-2 Vaccines) using the translation arsenal of the body. To date, Synthetic RNAs have been used in vaccines, gene editing projects, and several cancer treatments
However, to be effective, Synthetic mRNA needs to be administered to the body and find its way to the cell’s interior while evading the organism’s hostile environment towards RNA. In fact, RNA must avoid the host’s Immune responses, endonucleases, exonucleases and finally endosomes… to reach the inside of the cell without being destroyed or modified and have its intended effect. Early trials with bare RNA therapies proved inefficient due to poor intracellular delivery and disappointing results. Additionally, viral vectors were ruled out due to poor encapsulation efficiency, lower packing capacity, and higher immune reactions.
For this reason, one of the major enablers of novel RNA therapies in current development is the ability to encapsulate mRNA in a nano-capsule constituted by certain lipids forming a complex and new kind of non-viral delivery vehicle: a Lipid NanoParticle (LNP).
What are RNA-LNP?
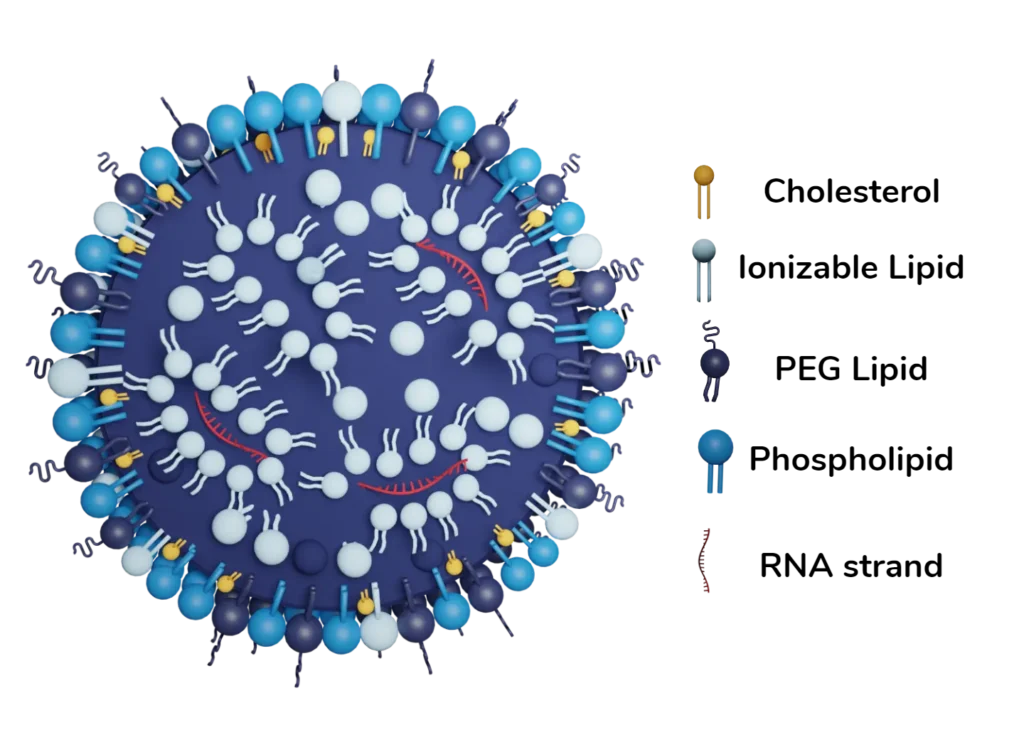
In brief, RNA LNPs often contain single-stranded piece of ribonucleic information surrounded by a layer of lipids chosen to target specific tissues and or organs. LNPs are very similar to the membrane of any cell but they do differ in one point: their composition especially in terms of proportions and nature of the lipids. For example, the relative amount of Phospholipid greatly differs compared to “regular” membrane cells’ wall. Interestingly, the precise composition of LNPs’ lipid layer must be tailored to the cargo (i.e what is being transported by the LNP) in order to optimize the tissues that are targeted and enhance tissue and organ tropism. The most important lipid portion being the ionizable lipid which helps improve RNA encapsulation, escape the immune system, and enter the cell. The detailed roles of each lipid component can be consulted in our review on LNP composition. [1]
Factors impacting biodistribution
As we have said, there are a good number of factors that come into play that might have an impact on biodistribution. One of the more obvious ones is the physical and chemical properties of the formulated nanoparticle such as the LNP size & ionization of the LNP. Other factors like the method used to formulate the RNA LNP and even where they are administered when given to someone might influence their biodistribution properties as well. Knowing and studying those factors is the first step towards a better understanding of targeting specific tissues and organs.
Size & ionization
Thanks to the ability to control the flow rate parameters (Total Flow Rates, Flow rate ratio…) in microfluidics formulation, specific LNP size can be more easily achieved. Meanwhile in manual mixing, the size of the particles are subject to variations caused by the experimental error or inconsistence in the mixing parameters. They will however not go lower than 20nm in size and the size obtained can be very different from one LNP formulation to another while still being in the nano-size range. Techniques used and formulation will give all kind of LNP sizes LNP are generally classified according to their size as it will greatly impact their biodistributions and clearance. Typically, we use the 3 following categories:
- Small Sized LNPs (~30nm) [2] : Thanks to their smaller size they can enter most tissue and have better biodisponibility, but they struggle to get out of the cells which augments toxicity. Furthermore, small sized LNPs have been shown to dissociate in the general circulation and lose some part of their lipidic structure which results in lesser protein expression in some cases.
- Medium sized LNPs (~100 nm) [3] : They tend to stick to their injection site but are still able to enter the circulation and reach the liver mainly via lymphatic nodes and by advection (meaning that they simply circulate in the fluid of the lymphatic system)
- Large sized LNPs (~300 nm) [4] : They also reach main circulation via Dendritic Cells in the lymphatic system not unlike the medium-sized LNPs. They suffer from shorter Clearance time in the circulation and tend to have less cellular uptake compared to smaller LNPs
Another aspect to consider is the overall charge of the LNPs. The LNPs are introduced in the cell thanks to the specific lipid structures of LNPs (Helper lipid, Ionizable lipid, Pegylated lipid and cholesterol) and found themselves in endosomes. The ionizable lipid portion of the membrane gives the ability to escape the endosomal environment in an acidic environment thanks to the subsequent change in charge of the lipid. [Link to Celine’s review]
Interestingly, Changing the ionizable lipid may change the biodistribution profile and how it’s integrated in tissues and organ. For example, substituting an ionizable lipid for an anionic lipid allows preferential delivery to the organs involved in the Reticulo Endothelial System [5]. Organs and tissues reached by LNPs will be elaborated further in the review
However, Size is linked to another very important factor: the mode of administration. Which will determine how the LNP will access the organism’s circulation and thus, how easy they will be able to find their way to certain organs.
Mode of administration
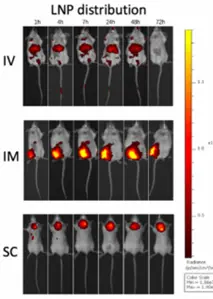
As previously said, the mode of administration plays a huge role in biodistribution. Choosing an administration route influences the tissues that will be first in contact with LNPs which will in turn affect where the cargo is delivered and where the LNPs will travel. There are 3 main choices when considering the administration route: Intramuscular Injections (I.M.), Intravenous Injections (I.V.) and Subcutaneous Injections (S.V.). Most information on biodistribution and organs reached by LNPs concerns the IM injection route because the RNA-LNP vaccines are administered that way. No matter the route chosen, LNPs are absorbed into the circulatory system and are present in most organs. However, Biodistribution studies mostly demonstrate weak and inhomogeneous signals in most tissues 48 hours following an injection [4].
- When injected intramuscularly: LNPs tend to stick to the injection site at first look but then slowly “leak” out the injection site into the circulatory system where they are then able to reach well-irrigated organs. LNPs presence has been shown in the circulation 15 mins after injection. 4 hours following the injection, LNPs are at a maximum in the plasma. In the next 48 hours, LNPs are found mainly in the liver, Adrenal gland, Spleen, and ovaries. When 48 hours have passed, 20% of the LNPs administered are found in the liver and other organs are mostly free of any LNPs. Protein Expression is slow and steady in the injection site but also rapid and “responsive” in the liver. [4]. No differences between males and females have been noted implying biodistribution profiles are independent of sex type. Furthermore, in the case of intramuscular injection of self-amplifying RNA (saRNA), replication has occurred more in the injection site than in the liver.
- When injected intravenously, LNPs find themselves directly into the main circulation which results in the same organs and tissues being reached by LNPs as IM injection. Indeed, they are found in the liver, the adrenal gland and the Spleen 1 hour after the injection. However, they will not be as persistent in the injection site as Intramuscular Injections.
- When injected Subcutaneously, similar patterns as Intramuscular injections have been found for mRNA LNPs.
Overall, LNPs will tend to stay at their injection site and depending on if this injection site is well irrigated (and drained by the lymphatic system), LNPs will be transported to other organs in the body which raises questions about the safety of certain proteins and drugs that may be created since they will eventually travel in the whole body and not only in the injection site;
Other methods of formulation for LNPs can be imagined such as an oral formulation for LNPs [6]. In fact, some of the lipids that are used for the formulation of the membrane are often based on dietary lipids so the Idea of an orally formulated LNP is not out of the question. However, the environment they would face in the Gastrointestinal (GI) tract is a very harsh one. Namely because the variations of pH would influence LNPs and their surface charges and thus their bioavailability would be reduced. Furthermore, the presence of digestive enzymes would be a great obstacle to the presence of LNPs in the GI tract. Additionally, the presence of microbes could have unintended effects on the LNP formulation altogether. Further studies need to be concluded and potentially highlight the impact of every factor that could influence RNA LNPs before the oral route can be considered as a solid route of administration.
Additionally, great efforts have been made to design LNPs that are meant to be released in nasal tissues to have its effect, enhancing the ease of use and avoiding an invasive mode of administration like injections. However, not much insight was acquired on the biodistribution on this mode of administration especially when considering its proximity with olfactory nerves and the central nervous system. Acquiring new information based on biodistribution studies is crucial if this mode of administration is to be elaborated.
The method of fabrication (Microfluidics, Manual Mixing)
While it is known that the LNP manufacturing process can impact the size of LNP nanoparticles, there are questions about the eventual impact of the technologies used to obtain LNPs. In fact, the shift towards new methods offering higher control of the nanoparticle characteristics, repeatability, higher encapsulation efficiency (namely microfluidics) has left many to ponder the differences that might exist between LNPs formulated with those new technologies especially in terms of organ tropism (I.e biodistribution) and mRNA delivery.
Microfluidics enables the production of LNPs in a continuous process, this allows control over experimental factors and permits repeatability and reproducibility as well. Aside from the formulation parameters (N:P ratio, concentration, relative proportion of each lipid) 2 synthesis factors are important in microfluidics: Flow Rate Ratio (FRR) and Total Flow Rate (TFR) as they have a direct impact on the size, PDI, EE%… of the produced nanoparticle. Each LNP, to have its intended delivery in the right organ, must have optimized these two factors to have the best passive targeting possible. Depending on the decided factors, RNA delivery efficiency can range from 100x worse to 4x better [7] than manual mixing highlighting the need to control and optimize those parameters as precisely as possible to obtain the best delivery possible.
Biodistribution, can also be somewhat impacted by the technique used for mixing but it’s unclear as to why this is happening. In some cases, spleens and livers exhibited different LNP percentages between manual formulation and microfluidic formulation of the same LNP. When targeting the liver, microfluidics should not affect the biodistribution all that much (Considering a majority of LNPs end up in the liver). However, if the targeted tissue is extrahepatic, extra care should be taken when using microfluidics for the formulation of LNP [7].
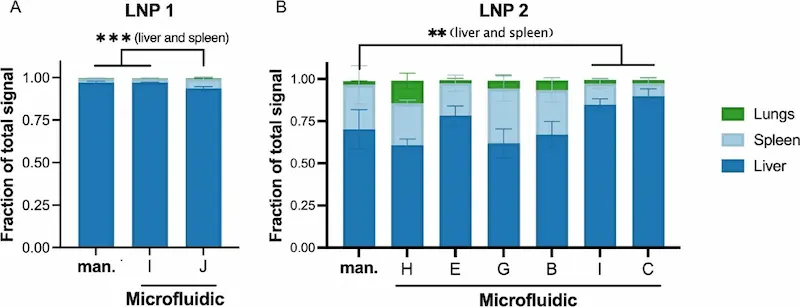
So, in short, Microfluidics has the potential to be better than manual mixing especially as control of the final nanoparticle characteristics in passive targeting is crucial for their efficiency. However, not controlling the parameters and not adapting them to lipid LNPs (FRR, TFR) can lead to reduced efficiency and potentially altered biodistribution.
Methods used to track LNP biodistribution profile
Different experimental methods can be used to track LNPs in the organism. Most studies are more interested in the final location of the cargo (E.g RNA) in the organism which is not a great indicator of where the LNPs are. In fact, molecules might be produced and then excreted because of LNP integration in certain cells and have different biodistribution profiles than the LNP itself (and thus be able to absorb onto different tissues compared to LNPs) [8]. Thus, it is important for theses experimental methods to properly track the molecules contained in the structural composition of the LNPs and not the molecules embedded inside the particle. . In the following paragraphs we will expose in short details the most used methods
In most cases, LNPs need to be labelled with a trackable molecule (Fluorescence, Radioactivity…) mostly because LNPs are not trackable by themselves (Although they can be directly observed thanks to Electronic Microscopy). Then, a specific method of detection is used to detect the labelled LNP which gives more or less accurate readings about the concentration/quantity of LNPs present in the sample. In the context of a biodistribution study, this often means that samples of blood, tissue and organs are analysed to find the presence of LNPs at specific times which ultimately give information about the distribution of LNPs in the whole organism. Thus, helping achieve a better picture of the global biodistribution of LNPs.
Stable isotope labelling
One method that is being used to track LNPs in the organism is the usage of Stable Isotope labelling (SIL). For example, Deuterium 2H can be used to replace “normal” hydrogen (1H) and fabricate deuterated cholesterol which allows production of 2H-labelled LNPs . Then, LNP concentration can be quantified following a Liquid Chromatography/ Mass spectrometry analysis which is able to identify and distinguish the deuterium from LNPs in biological samples (2H being “heavier” than 1H, it is easily distinguishable from 1H)
Fluorescent labelling
Fluorescent labelling is also a technique that may be used as long as the fluorescent molecule/dye is specific (or specifically attached) to the LNP structure. In this technique, Fluorescent component are included into the formulation (I.g fluo-DOTAP) Commercial kits containing fluorescent cholesterol [9] are currently being sold and offer an easy and practical way to analyse LNP uptake in cells. Fluorescent labelled antibodies could also technically be used but they may alter passive targeting and biodistribution. Such kits and molecules can be used to track LNPs in the body. After Injecting LNPs in model animals, Fluorescence can be followed either by analysing samples directly (Fluorometer) or by using live in-vivo imaging which provides images at different timepoints. Live in-vivo imaging accurately shows where the fluorescence is in the organism and also gives an idea of relative concentration of the analytes in the organism. This method does require anatomical knowledge of the model animal and is better used when correlated with other techniques to draw out scientific conclusions.
Radiolabelling
Radiolabelling is also a possible method that may be applied in the case of LNPs. In this method, Labelling is realised with a radioactive molecule such as 64Cu[10]. Radioactivity, or rather the annihilation of two positrons, is then detected thanks to Positron Emission Tomography (PET) Scanning. Radiolabelling is an expensive method and needs access to also expensive equipment, but it gives good data.
Others
The advantages and disadvantages of most medical imaging used to track nanoparticles are further explicated in This chart from the article “A review on the role of lipid-based nanoparticles in medical diagnosis and imaging” by Mozhdeh Mirahadi, Saeed Ghanbarzadeh, Marjan Ghorbani, Amin Gholizadeh & Hamed Hamishehkar published in 2018.
| Modality | Advantage | Disadvantage |
|---|---|---|
| MRI | – Noninvasive – Evaluating anatomy and function of tissue – High spatial resolution – Provide good soft tissue contrast | – Low sensitivity – Expensive equipment |
| CT | – High-sensitivity anatomical information – 3D imaging | – Limited functional information – Poor soft tissue contrast – Expensive equipment |
| US | – Safe – Low cost – Real-time measurement | – Unable to differentiate between tissues with similar acoustical properties – Low sensitivity and resolution |
| NIR | – Deep tissue penetration – Low tissue absorption and scattering – Minimal auto fluorescence | – Low sensitivity – Problems in aqueous solutions |
| PET | – Provide biochemical information – High sensitivity – 3D imaging | – Limited anatomical information – Expensive equipment |
| SPECT | – Detecting multiple probes simultaneously | – Low sensitivity |
LNPs and how they diffuse into the organism: main organs and tissues
Once they are inside the circulatory system and depending on the mode of administration. LNPs will preferentially always reach the organs of the reticulo-endothelial system (RES). This means that LNPs are mainly found in the liver but also in other main organs such as the heart, the spleen or even the lungs. Potentially, LNPs can reach every irrigated tissue albeit not homogenously and not in the same proportions, they will however always reach the liver to be metabolised.
The RES is involved in the homeostasis of the circulatory system and is formed mainly by the liver (90%) and more precisely certain cells contained in the liver: Kuppfer Cells (KCs) and Liver Sinusoidal Endothelial Cells (LSECs). It is thought that LNPs in the circulation adsorb to ApolipoproteinE which permits entry to a Low-Density Lipoprotein (LDL). The Lipoprotein will carry its content to the RES in the cells previously mentioned are able to phagocyte certain macromolecules such as the LDL and hyaluronic acid and thus they integrate the cells.
This explains why LNPs are found throughout the body but mostly in certain organs and tissues like the spleen or the liver… However, Lipid composition of the LNP plays indubitably a role in biodistribution. For example, simply switching a zwitterionic lipid to an anionic lipid modifies the mechanism of interaction with cells and, hence, the biodistribution profiles as we have seen in this review.
However, some tissues remain harder to reach than others: this is the case for the Central Nervous System that is protected by the Brain Blood Barrier (BBB) and possibly prevents LNP transportation to the brain. Some studies highlight that Brain distribution of LNPs is possible but needs special ionizable lipids to be able to get through the blood brain barrier and deliver their cargo into brain cells . Furthermore, small evidence shows that products of mRNA LNPs might be able to cross the blood/Breast milk in small quantities, but clinical implications have not been thoroughly assessed as of now.
Conclusion on RNA-LNP biodistribution
LNP biodistribution is critical for the success of mRNA therapeutics, and more generally RNA therapeutics, and remains a subject that deservers careful attention as the mechanism involved in the biodistribution of LNPs have been identified to be related to chemical and physical properties. More information is needed on organs and tissues that are protected by additional barriers such as the brain. The actual pathway that leads to the biodistribution patterns of LNP is not entirely elucidated too, while the RES is certainly involved via the LDL/Apolipoprotein E. More studies need to be done to explain why different formulations and techniques may result in different biodistribution profiles.
Despite advances, tissues protected by barriers like the BBB require innovative lipid formulations for effective delivery. Until biodistribution mechanisms are fully understood, empirical screening of Lipid Nanoparticles remains the most reliable approach for evaluating LNP performance in vivo. Future studies should focus on elucidating the pathways and barriers that govern biodistribution to improve therapeutic outcomes.

Looking to improve the control & reproducibility of your LNP formulation?
Reach out to us to learn how we can help!
References
[1] « RNA-LNP vaccines: The Role of Lipid Nanoparticle Compositions – Inside Therapeutics ». Checked on June 24th 2024. Available at: https://insidetx.com/resources/reviews/rna-lnp-vaccines-the-role-of-lipid-nanoparticle-compositions/
[2] S. Chen, Y. Y. C. Tam, P. J. C. Lin, M. M. H. Sung, Y. K. Tam, et P. R. Cullis, « Influence of particle size on the in vivo potency of lipid nanoparticle formulations of siRNA », Journal of Controlled Release, vol. 235, p. 236‑244, août 2016, doi: 10.1016/j.jconrel.2016.05.059.
[3] J. Christensen et al., « Biodistribution and metabolism studies of lipid nanoparticle-formulated internally [3H]-labeled siRNA in mice », Drug Metab Dispos, vol. 42, no 3, p. 431‑440, mars 2014, doi: 10.1124/dmd.113.055434.
[4] J. Di et al., « Biodistribution and Non-linear Gene Expression of mRNA LNPs Affected by Delivery Route and Particle Size », Pharm Res, vol. 39, no 1, p. 105‑114, janv. 2022, doi: 10.1007/s11095-022-03166-5.
[5] R. Pattipeiluhu et al., « Anionic Lipid Nanoparticles Preferentially Deliver mRNA to the Hepatic Reticuloendothelial System », Adv Mater, vol. 34, no 16, p. e2201095, avr. 2022, doi: 10.1002/adma.202201095.
[6] M. Plaza-Oliver, M. J. Santander-Ortega, et M. Victoria. Lozano, « Current approaches in lipid-based nanocarriers for oral drug delivery », Drug Deliv Transl Res, vol. 11, no 2, p. 471‑497, 2021, doi: 10.1007/s13346-021-00908-7.
[7] D. M. Strelkova Petersen, N. Chaudhary, M. L. Arral, R. M. Weiss, et K. A. Whitehead, « The mixing method used to formulate lipid nanoparticles affects mRNA delivery efficacy and organ tropism », European Journal of Pharmaceutics and Biopharmaceutics, vol. 192, p. 126‑135, nov. 2023, doi: 10.1016/j.ejpb.2023.10.006.
[8] M. R. Edelmann, « Radiolabelling small and biomolecules for tracking and monitoring », RSC Adv, vol. 12, no 50, p. 32383‑32400, doi: 10.1039/d2ra06236d.
[9] « LipidLaunchTM LNP-0315 Uptake Kit (Green Fluorescence) ».
[10] P. Wong et al., « PET Imaging of 64Cu-DOTA-scFv-Anti-PSMA Lipid Nanoparticles (LNPs): Enhanced Tumor Targeting over Anti-PSMA scFv or Untargeted LNPs », Nucl Med Biol, vol. 47, p. 62‑68, avr. 2017, doi: 10.1016/j.nucmedbio.2017.01.004.