A comprehensive review of passive and active nanoparticle targeting technics

Nanoparticle targeting is revolutionizing the gene therapies and vaccine field, offering unprecedented precision in drug delivery by directing cargos to specific cells or organs in the body. This article dives into the intricacies of passive and active nanoparticle targeting strategies, comparing their mechanisms and applications. We explore how these advanced strategies enhance treatment efficacy, opening new frontiers in medical science.
Introduction to Gene Therapies
Gene therapy is an emerging field in medical and pharmaceutical sciences because of its potential in treating chronic diseases like cancer, viral infections, myocardial infarctions, and genetic disorders.
However, application of gene therapy is limited because of lack of suitable methods for good introduction of genes into cells. Current gene therapies in clinics are mostly based on viral vectors, especially AAVs (adeno associated virus). Even though the viral agents have a high transferring efficiency, they are difficult to handle due to their toxicity, their immunogenicity, the inability to perform repeated dosing, limited package capacity and high associated costs.
Recently, non-viral vectors have emerged, especially in RNA therapy, to overcome viral vectors’ limitations. Several nonviral in vitro and in vivo gene delivery systems are developed. Out of these, two main classes of biomaterials have been explored to develop non-viral delivery vectors—lipid and polymeric nanoparticles (NPs).
Nanoparticles Biodistribution
The fate of nanoparticles in the body after intravenous administration (IV) is a major obstacle encountered by scientists when administering Drug Delivery Systems in vivo.
Liver tropism
The liver is commonly identified as the main site where nanoparticles gather and are removed from the system, indeed 60 to 90% of the NPs injected in the body by IV are directed in the liver. This phenomenon called “Liver Tropism” is due to the liver's extensive blood capacity and complex vasculature. For instance, nanoparticles traveling at speeds of 10–100 cm/s in arteries and veins can significantly slow down to 200–800 µm/s in liver sinusoids [1]. Consequently, numerous circulating Nanoparticles experience an extended residence time in the liver.
The rest of the nanoparticles that have escaped the liver are mainly found in the spleen (2-20%) or in the bone marrow (1%). Only a few NPs really reach their main target.
Immune response
Nanoparticles can be recognized as foreign bodies. Indeed, the hepatic accumulation of NPs is followed by the clearance mechanism of the RES (reticuloendothelial system) which constitutes first line of immune defense, and that contains liver-resident macrophages (Kupffer cells: 90% of the macrophage cells of the organism) and liver sinusoidal endothelial cells (LSECs) [1].
This RES, also known as the mononuclear phagocytic system (MPS), promotes the removal of particles from circulation within seconds to minutes using surface opsonization.
Opsonization is an immune process which uses opsonin proteins to tag foreign pathogens for elimination by phagocytes.
How can nanoparticle targeting improve delivery efficiency?
As described on the below schematic, there are 2 main approaches to nanoparticle targeting: either active or passive: In a nutshell, passive targeting consists of tuning nanoparticle characteristics to improve its specificity while active targeting involves incorporating targeting elements into the delivery vehicle to enhance its deliverability to the target cell/organ.
![Lipid-based-nanoparticle-active-and-passive-targeting Schematic describing the available active and passive-targeting strategies for Lipid-based-nanoparticle [7]](https://insidetx.com/wp-content/uploads/2024/04/Lipid-based-nanoparticle-active-and-passive-targeting-730x537-1-730x537.webp)
Passive Targeting of Nanoparticles
Definition of nanoparticle passive targeting
Passive targeting is a strategy accomplished by integrating the therapeutic agent into a nanoparticle, allowing it to passively diffuse in the body in the aim to reach the target organ/tissues. In the context of gene therapy, passive targeting is based on nanoparticles (NPs) to precisely direct therapeutic agents to specific tissues or organs, without the need for specific targeting agents [2],[3].
This approach takes advantage of the unique physiological or pathological features of the targeted region, facilitating the preferential accumulation of NPs and enabling targeted drug release. It is important to look at organ physiology to determine the ideal size and load for nanoparticle penetration and accumulation in target organs or tissues. Here are a few examples of the capillarity/permeability of the vascular wall, depending on the tissue or organ.
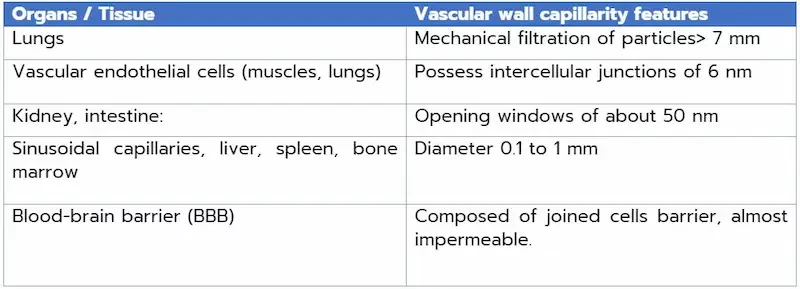
To optimize nanoparticle targeting and take advantage of passive targeting, it is interesting to adjust nanoparticle size and composition. These modifications improve their circulation in the body, promote efficient accumulation in specific targeted organs and thus maximize therapeutic impact.
Nanoparticle Characteristics influencing Passive Targeting:
Nanoparticle Size
Passive targeting, essentially achieved by diffusion transport, is highly dependent on nanoparticle size. An optimal size of 20 to 200 nm favors prolonged circulation, increased accumulation in tumors/organs, and reduced renal elimination (NPs < 7nm). A size between 20 and 150 nm reduces hepatic clearance and kidney filtration. [6]
In addition, smaller lipid nanoparticles benefit from prolonged circulation and enhanced accumulation in target tissues thanks to the enhanced permeability and retention effect (EPR), escaping immune surveillance.
EPR effect is the ability to diffuse passively through porous vascular walls. The principle of the EPR effects was recently accepted as a passive targeting strategy for nanoparticles tumor retention and accumulation. Indeed, tumors exhibit enhanced vascularization with significantly dilated vessels. The wider pores between endothelial cells in tumor capillaries facilitate the extravasation of the drug delivery systems and good leverage of the NPs, in contrast to the tighter structure observed in normal capillaries.
Lipid Composition
Lipid composition can also be improved for an optimized biodistribution of nanocarriers. Let's talk about liposomal doxorubicin for example. Also known as Doxil®, it is the first FDA-approved nanomedicine, based on encapsulation of the treatment in a liposome consisting of hydrogenated soy phosphatidylcholine (mainly DSPC), cholesterol and PEG-lipid in a well-determined molar ratio. This formulation is the result of nearly two decades of optimization to achieve the desired particle properties: enhanced encapsulation efficiency, extended circulation time to exploit the enhanced permeability and retention (EPR) effect, improved drug accumulation at the target site and fewer dose-limiting toxicities. [6]
For nucleic acid delivery, the large size and high negative charge density require additional lipid functionalities, resulting in current LNP formulations containing ionizable lipids, phospholipids, cholesterol and PEG-lipid.
Discovered in the 1990s, ionizable lipids such as the remarkable MC3 are a major asset in the formulation of lipid nanoparticles. Their essential role in the efficient encapsulation of nucleic acids, illustrated by the success of Onpattro®, underlines their practical relevance in RNA therapy. [6]
The impact of ionizable lipids on tolerance and immunogenicity is particularly apparent in mRNA vaccines, where pKa, surface charge and lipid composition have a significant influence on immune response.
The pH-dependent charge modulation of ionizable lipids plays a crucial role in intracellular drug delivery, responding to pH changes during endocytosis. This characteristic facilitates the efficient release of drugs inside the cell by endosomal escape. In addition, the unique ability of ionizable lipids to return to a neutral charge state in physiological pH environments contributes to the overall safety of the drug delivery system. Improved drug loading capacity further enhances the safety of the delivery system and reduces the risk of toxicity and poor biodistribution of the nanocarrier and its contents.
Surface Characteristics
Surface properties exert a significant influence on the degree of internalization of nanoparticles (NPs) into cells. Surface modification can be achieved by polymer composition, adding an extra dimension of hydrophobicity or hydrophilicity to the particles. PEGylation (adding polyethylene glycol) Is one of the revolutionary solutions found to avoid several problems of biodistribution of drug delivery systems. Indeed, PEGylation of nanoparticles helps to escape opsonization and at the same time problem of RES clearance.
Ultimately, this solution prolongs the half-life of nanoparticles. And in addition, increasing the molecular weight of hydrophilic polymer chains will prolong the circulation time of these NPs. As a result, NPs can finally reach their target tissue organ and deliver their drug.
In the context of gene therapy and lung diseases such as cystic fibrosis, for example, mucus-lined lung tissue is a major barrier to nanoparticle penetration and delivery of the nanocarrier of RNA to its target in the lungs. But thanks to optimized PEG molarity (and PEGylation) and sterol structure, LNPs efficiently deliver mRNA to cystic fibrosis lungs across mucosal barriers.
How to optimize nanoparticle characteristics for passive targeting in Practice?
It is necessary to carry out a rigorous selection stage of suitable lipids for the formulation of lipid nanoparticles. This involves a screening process, during which different lipid compositions (different phospholipids, different ionizable lipids, different lipid concentrations and ratios) are evaluated to select those with the desired properties and characteristics for the nanoparticles. This selection process is crucial to ensure the quality, efficacy and safety of the drug delivery system.
Nanoparticle manufacturing processes play an essential role in the synthesis and characterization of lipid nanoparticles. Different approaches, such as solvent evaporation or high-pressure homogenization, often result in insufficient control of critical characteristics due to their mixing process, leading to large nanoparticle distribution (PDI) and poor reproducibility.
To overcome these difficulties, innovative microfluidic-based synthesis techniques are being developed. Microfluidic mixing processes enable precise control of conditions, producing more uniform LNPs with controlled sizes and improved repeatability. Mixing speed, flow rates and ratios between organic solutions and aqueous solutions influences particle size, enabling fine-tuning of synthesis parameters and guaranteeing reproducibility. TAMARA is our nanoparticle production platform, enabling us to produce lipid nanoparticles from 0.2 ml to 10 ml quickly and easily.
Then multiple tests are done to characterize these nanocarriers to acquire information about the size, the charge, the PDI (using Dynamic Light Scattering for example) and control of encapsulation efficiency (EE%), and choose the best lipid composition, the best parameter et the best features for the small drug delivery system.
Looking to synthesize nanoparticles using microfluidics?
Discover our innovative and user-friendly nanoparticle synthesis platforms
Active Targeting of Nanoparticles
To overcome the limitations of passive targeting, active targeting has recently been emphasized.
Definition of nanparticle active targeting
Active nanoparticle targeting involves incorporating targeting elements. It is mainly divided into antibody-based targeting, peptide-based targeting, aptamer-based targeting, and small-molecule-based targeting. This strategy aims to guide nanoparticles specifically to cells, tissues, or organs by exploiting unique physiological or pathological features of the target region.
Once the nanoparticles reach their target, they can interact specifically with target cells, facilitating the targeted delivery of drugs, genes, or other therapeutic agents encapsulated in the NPs.
This approach aims to improve treatment efficacy and specificity while minimizing adverse effects on surrounding healthy tissue [2],[3].
Nanoparticles Functionalization:
Nanoparticle-based drug delivery faces complex challenges as it navigates the body. Although these small vectors have huge potential, certain physiological barriers and impermeable membranes within the body present obstacles to their effective delivery. Passive targeting, as we saw earlier, relies on the intrinsic characteristics of nanoparticles for barrier-free delivery. However, this strategy faces limitations, particularly when it comes to administering therapeutic agents in highly protected regions such as the central nervous system (CNS), where the blood-brain barrier (BBB) constitutes a formidable defense. Thus passive targeting is sometimes inefficient to deliver nanotherapeutics to the central nervous system (CNS). This limitation is due to the almost impermeable nature of the BBB. The remaining solution is active targeting. And one of the most used techniques involves the use of antibodies that specifically recognize an antigen present on BBB cells. [1] Up to now, there have been few examples of non-viral delivery to the brain, but they have been innovative.
Another way to cross the BBB is through receptor-mediated transcytosis. By using ligands against the receptors at the apical membrane of BBB endothelial cells (such as transferrin, insulin, leptin and lipoproteins), apical-to-basolateral cargo transport can be achieved.
Concerning Tumor cells, it has been shown that preferentially express certain proteins or receptors at their surface. For instance, the protein Ligand PD-L1 (Programmed Death-Ligand 1) is overexpressed in tumor cells. its role is to bind the PD-1 receptor (Programmed Cell Death Protein 1) to the surface of immune cells (LT cells) and attenuate the immune reaction (by inhibiting LT activity), so that immune cells escape the immune system and proliferate indefinitely. This reaction can be exploited by functionalizing nanoparticles with PD-L1 receptor that will target tumor cells and deliver RNA/molecules that will destroy the cell or interrupt proliferation and unleash the immune response to attack cancer cells. [5]
Active targeting assembly methods
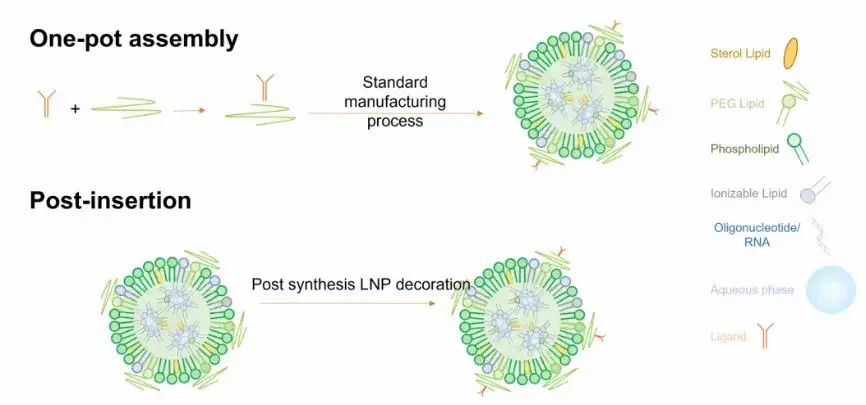
Targeted lipid nanoparticles can be generated through a one-pot assembly process or by post-insertion process.
One-pot assembly:
In this process, targeting elements are initially mixed with structural lipids, followed by assembly methods to create targeted lipid nanoparticles. These assembly methods include evaporation, thin film hydration, microfluidic mixing, and ethanol injection.
Nevertheless, this method presents some drawbacks, indeed over 50% of small targeting ligands were found to be positioned within the cavity of lipid nanoparticles, making them inaccessible for active targeting. Moreover, larger molecular ligands like antibodies and aptamers, are expensive and susceptible to fragility during fabrication conditions, such as exposure to organic solvents, high temperatures, or mechanical forces [7]. As a result, the synthesis yield for targeting ligand-lipid conjugates is very low.
Post-insertion Assembly:
Given these considerations, the typical approach involves the postinsertion of large and delicate targeting ligands into preformed, unmodified lipid nanoparticles. Initially, a plain lipid nanoparticle was created using methods akin to one-pot assembly. Subsequently, its surface is adorned with functional groups capable of reacting with targeting ligands through surface conjugation. In this scenario, targeting ligands are exclusively located on the outer surface of lipid nanoparticles, with the density and functionality being adjusted by the efficiency of surface conjugation.
However, studies have shown that lipid targeting nanoparticles prepared by post-insertion bind late and less to target cells compared to those prepared using a one-step assembly process for the same purpose. This highlights the limitations of surface functionalization in the preparation of lipid targeting nanoparticles and highlighting its inefficiency and reduced stability.
Comparison of nanoparticle Passive and Active Targeting approaches
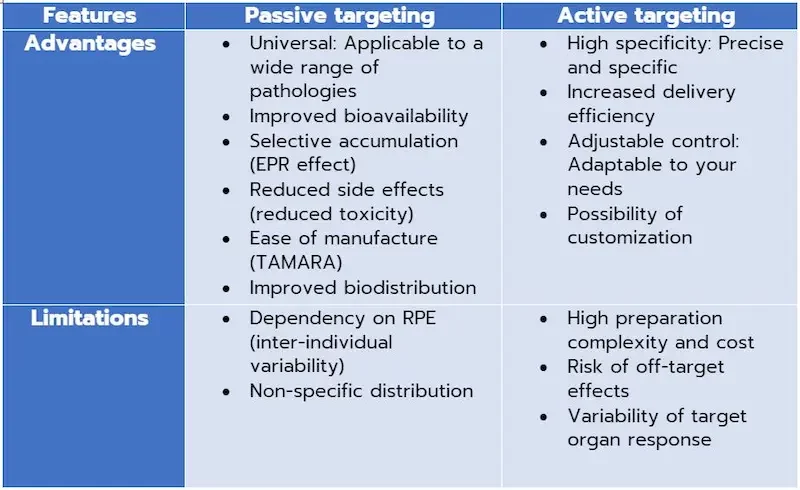
Targeting nanoparticles is very delicate and really depends on the pathology and the targeted tissue/organ. In the context of gene therapy, passive targeting uses the enhanced permeability and retention effect (EPR) to facilitate the selective accumulation of gene therapy vectors at target sites. This universal approach is applicable to a variety of diseases, offering improved bioavailability, increased concentration in affected areas and reduced adverse effects. In terms of production, the simplicity of manufacturing passively targeted nanoparticles represents a very important asset, as microfluidic techniques such as TAMARA enable these nanocarriers to be produced in no time at all, and with a quality that guarantees efficacy.
Active targeting in gene therapy involves modifying nanoparticle surfaces with specific ligands, enhancing the specificity of therapeutic vector delivery. While this offers high specificity, enhanced delivery efficiency and adaptability to meet specific therapeutic needs, active targeting can become complex and costly due to the requirements involved in selecting and modifying targeted molecules (not to mention the price of targeting elements such as antibodies and aptamers, for example). Challenges such as off-target effects and variations in target organ response can also influence its effectiveness.
It Is true that active targeting has its drawbacks, but so does passive targeting, such as dependence on RPE, which can vary according to disease type and individual. Indeed, some tumors may not exhibit a sufficiently high EPR, limiting the targeting and addressing efficiency of NPs. In addition, inter-individual variability is also a factor: patients' physiological characteristics, such as tumor vascularization, can vary from one individual to another, which can affect the effectiveness of passive targeting.
Non-specific distribution: Although passive targeting can improve drug concentration in target tissues, a certain amount of non-specific distribution to other organs may still occur. This lack of specificity can lead to low bioavailability and secondary toxicity caused by low selectivity.
However, in active targeting, it is important to note that the selection and modification of target molecules can become more complex, leading to an increase in the complexity and cost of the preparation. In addition, there is a risk of off-target effects, and variability in target organ response can influence overall delivery efficacy.
Conclusion on nanoparticle targeting strategies for gene therapies
In a nutshell, gene therapy is currently at the forefront of innovation. The use of nanoparticles represents a promising approach to overcoming the obstacles associated with gene delivery. Depending on their target, these nanoparticles are confronted with several barriers as they make their way through the body. The most common biodistribution problems encountered are liver retention, opsonization, clearance from the RES and the permeability properties of different organ tissues. This has led to the development of targeting solutions. Passive and active targeting confront each other.
Passive targeting offers an effective and practical approach to drug delivery via nanoparticles. Its advantages include extended nanocarrier half-life, improved bioavailability, selective accumulation in targeted areas, enhanced efficacy and reduced side-effects. In comparison, active targeting, which is highly interesting and more specific, presents complex challenges linked to nanoparticle functionalization. By opting for passive targeting, at Inside Tx we are maximizing treatment efficacy while simplifying production with our manufacturing system, TAMARA. The aim of Inside Tx is to make clinical implementation more accessible, faster and more efficient.
Looking to improve your nanoparticle delivery?
Speak to our application scientist
References
[1] Alavi, Mehran, et Mehrdad Hamidi. « Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles ». Drug Metabolism and Personalized Therapy 34, no 1 (26 mars 2019). https://doi.org/10.1515/dmpt-2018-0032.
[2] Clemons, Tristan D., Ruhani Singh, Anabel Sorolla, Nutan Chaudhari, Alysia Hubbard, et K. Swaminatha Iyer. « Distinction Between Active and Passive Targeting of Nanoparticles Dictate Their Overall Therapeutic Efficacy ». Langmuir 34, no 50 (18 décembre 2018): 15343‑49. https://doi.org/10.1021/acs.langmuir.8b02946.
[3] Guo, Feng, Junfeng Ke, Zhengdong Fu, Wenzhao Han, et Liping Wang. « Cell Penetrating Peptide-Based Self-Assembly for PD-L1 Targeted Tumor Regression ». International Journal of Molecular Sciences 22, no 24 (10 décembre 2021): 13314. https://doi.org/10.3390/ijms222413314.
[4] Hald Albertsen, Camilla, Jayesh A. Kulkarni, Dominik Witzigmann, Marianne Lind, Karsten Petersson, et Jens B. Simonsen. « The Role of Lipid Components in Lipid Nanoparticles for Vaccines and Gene Therapy ». Advanced Drug Delivery Reviews 188 (septembre 2022): 114416. https://doi.org/10.1016/j.addr.2022.114416.
[5] Kim, Jeonghwan, Yulia Eygeris, Renee C. Ryals, Antony Jozić, et Gaurav Sahay. « Strategies for Non-Viral Vectors Targeting Organs beyond the Liver ». Nature Nanotechnology, 27 décembre 2023. https://doi.org/10.1038/s41565-023-01563-4.
[6] Li, Jianmin, Qingluo Wang, Guoyu Xia, Nigela Adilijiang, Ying Li, Zhenqing Hou, Zhongxiong Fan, et Jinyao Li. « Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy ». Pharmaceutics 15, no 9 (29 août 2023): 2233. https://doi.org/10.3390/pharmaceutics15092233.
[7] Bertrand, Nicolas et al. “Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology.” Advanced drug delivery reviews vol. 66 (2014): 2-25. doi:10.1016/j.addr.2013.11.009
