Lipid Nanoparticle for mRNA delivery
In the realm of cutting-edge medical advancements, the use of lipid nanoparticles for mRNA (messenger RNA) delivery have emerged as a groundbreaking tool for a new generation of therapeutics, as illustrated by the nobel prize awarded to Katalin Karikó and Drew Weissman for their work in the field. This revolutionary technology holds the potential to transform the landscape of medicine by enabling targeted and efficient delivery of genetic material. In this article, we delve into the remarkable capabilities of lipid nanoparticles, exploring their role in revolutionizing the field of mRNA delivery and its far-reaching implications for the future of healthcare
mRNA-LNP vaccines: In the spotlight during the Covid-19 pandemic
Following the emergence of the unprecedented Covid-19 disease, a considerable amount of Research and Development resources has been invested to develop an efficient Covid 19 vaccine to slow down its spread.
Numerous approaches have been investigated, including inactivated viruses, recombinant proteins, and influenza virus vectors, DNA vaccines as well as the revolutionary LNP encapsulated mRNA, which quickly appeared as the leading candidate for vaccine development. [1]
This innovative technology is based on the delivery, by means of a lipid nanoparticle, of a nucleic acid (mRNA) to the target cell of the human host. The host cell will by itself fabricate the target protein or peptide and express the antigen to trigger the fabrication of the corresponding antibodies. This allows the host immune system to react more quickly once exposed to the actual disease and prevents the latter from developing further.
mRNA-LNP therapies and vaccines outside Covid-19
Considering the success of the mRNA-based vaccine for infectious disease, both in terms of safety, efficacy and induction of B and T-cell responses, this novel technology has also been investigated – and showed very promising results - as an option for the development of novel vaccines against cancer and auto-immune diseases.
In the case of cancer therapies, messenger RNA vaccine strategies combine a genetic approach together with innovative immunotherapies targetting tumors (e.g Keytruda). Numerous tumors, often referred to as 'cold tumors,' manage to evade detection by the patient's immune system. The aim is to modify the cancer cells in a way that enables the immune system to recognize specific tumor antigens and eliminate them effectively.
For instance, recent studies have explored the use of mRNA vaccines, specifically those utilizing dendritic cells (DCs), in the context of melanoma treatment. Current research is focused on the development of multiple dendritic cells based mRNA vaccines designed to stimulate immune responses against a range of tumor-associated antigens (TAAs) in melanoma patients.
However, a major challenge lies in delivering immunotherapeutic molecules, as they must interact with various cell types to trigger an effective antitumor immune response. This is where scientists have turned to the use of nanoparticles (NPs) as carriers for cancer vaccines. Several approaches have been explored, yielding promising results in preclinical studies. These formulations typically combine a well-known antitumoral active pharmaceutical ingredient (API), such as doxorubicin or paclitaxel, with a molecule that promotes the activity of the immune system, as illustrated in our breast cancer nanotherapy article.
While offering multiple advantages over traditional vaccines, several technical challenges associated with effective mRNA vaccine development remain, the most important of which is to ensure the proper delivery of mRNA to the target cell cytoplasm, and the manufacturing of the appropriate vessel for its delivery.
mRNA delivery barriers
To maximize vaccine efficacy, an efficient delivery of the messenger RNA within the cell is needed.
To this end, several hurdles should be overcome:
- Following its administration, mRNA may be quickly degraded by the nuclease in physiological fluids. The RNA stability and protection from degradation is therefore mandatory.
- mRNA being a large molecule, the proper carrier should ease its crossing of the cell membranes, to reach the cytoplasm where translation occurs. [3]
An optimal mRNA delivery method should thus help keep it stable, protect it during the delivery process, and cross the cellular membranes to deliver it within the cells efficiently.
mRNA delivery methods
To optimize mRNA delivery, multiple methods have been tested, starting with the most basic one: injecting naked mRNA into the body.
Naked mRNA injection
The simplest administration strategy consists in the direct injection of free mRNA solution to the body. Multiple in-vitro and in-vivo tests and even clinical trials have been carried out
Although it cannot directly cross the cellular membrane, an internalization mechanism was shown to happen – most likely due to hydrostatic pressure – leading to a partial mRNA expression.
However, this method has quickly been ruled out as the internalization efficiency remained extremely low, and mRNA was highly susceptible to RNase degradation, decreasing its half-life when traveling in the bloodstream. This restricts the naked mRNA delivery to local applications, meaning that other delivery systems should be explored to better protect mRNA.
Viral vectors
Delivery of mRNA by viral vectors – such as engineered adeno-associated viruses – has also been explored. Viruses are genetically modified to integrate therapeutic genes partially or fully.
This offers the major advantage of being directly translated into the cytoplasm by the host ribosomes. However, despite their obvious interest, viral vectors show major drawbacks such as possible host rejection and genome integration among others thus leading to the need for non-viral vectors for RNA delivery. [4]
Polymer-based nanoparticles
The first kind of non-viral vector tested to better protect the mRNA from RNase degradation were polymer material such as PEI, PAMAM…, which have been tested to encapsulate mRNA, in HIV mRNA vaccines for instance.
While proving to be efficient for the protection of mRNA when transiting in the bloodstream, polymer-based nanoparticles showed possible high toxicity, mostly due to high polydispersity and positive charge of the polymers, and relatively poor delivery efficacy, which led to the exploration of other delivery methods
Lipid-based nanoparticle
Thanks to their improved biocompatibility compared to polymer, lipid-based nanoparticles quickly came up as an alternative solution. To this end, the first generation of nanoparticles was tested: the liposomes.
Liposomes consist of a lipidic bilayer shell with an aqueous core, which mimics the cell membrane structure and offer an efficient in-cell delivery of both hydrophilic (in the core) and hydrophobic (in the shell) small molecules and drugs. Liposomes are already widely used in the industry, for instance, the first approved liposomal drug was Doxil in 1995, a liposomal formulation of the antitumor agent doxorubicin [2]
However, due to the negatively charges nature of the mRNA, liposome showed poor encapsulation efficiency, and poor intracellular delivery of it. For this reason, a more complex lipid-based nanoparticle was engineered for the specific delivery of RNA within cells: the LNP.
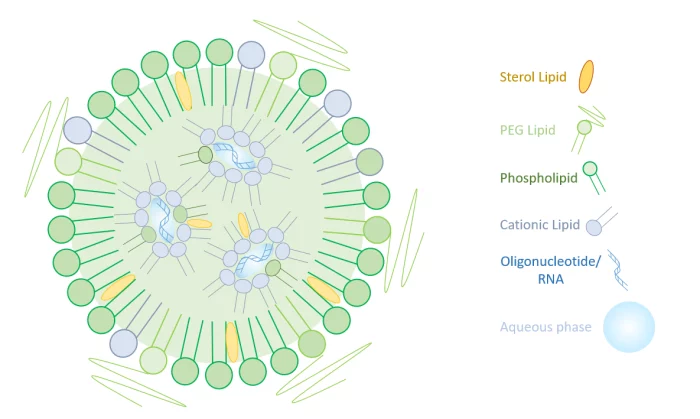
Lipid Nanoparticle for mRNA delivery
To overcome the limitation of the simpler liposomes, more complex lipid-based nanoparticles have been introduced specifially for the delivery of RNA. Based on a ionizable lipid, LNPs (Lipid Nanoparticles) have a unique set of characteristics that overcome all the limitations posed by the previously introduced delivery methods.
Ionizable lipids have the unique capability of changing charge with pH: They are positively charged at low pH – which helps mRNA complexation in acidic buffer during synthesis, as well as its release during endocytosis – and neutral at physiological pH - to reduce toxicity.
The combination of this cationic lipid with a sterol lipid, a phospholipid, and a PEG allows for efficient encapsulation of mRNA while protecting nucleic acids from degradation in bloodstream and aiding cellular uptake, thus offering excellent in-vivo transcription efficacy.
Check here for the detailed lipid nanoparticle composition of the commercially available RNA-LNP therapeutics.
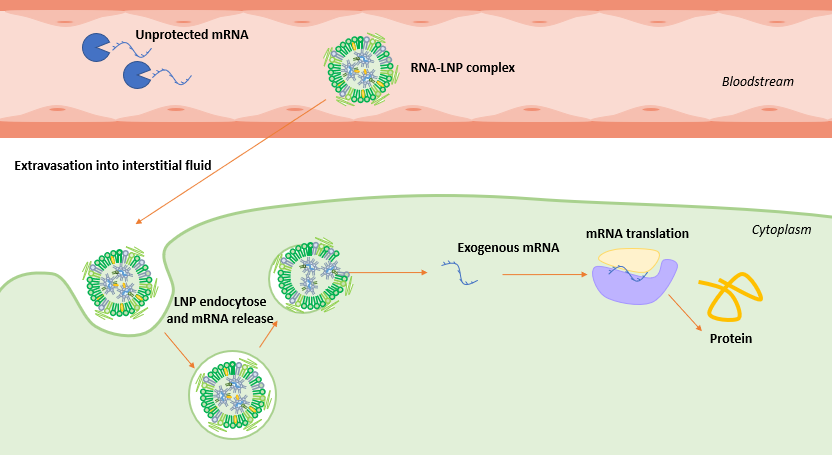
mRNA-LNP delivery mechanism
For the therapy to be successful, enough mRNA should enter the cell to trigger the synthesis of the target protein, however its journey to the cytoplasm is long and tedious.
Following its injection, mRNA-LNP complex travels through the host bloodstream, with the mRNA protected by the lipidic membrane to help increase its half-time, until reaching the cell surface.
As the mRNA cannot cross the cellular membrane by mere diffusion, the mRNA-LNP complex binds to the cell receptors. They are then taken up by the cell into the endosome, an isolated, low pH, compartment within the cell. As the mRNA LNP complex has been internalized, the drop of pH in the endosome leads to a decrease in its stability, triggering the delivery of the mRNA, which escapes its lipidic compartment to reach the cytoplasm.
Once released, like any other mRNA strand present in the cell cytoplasm, the exogenous mRNA gets translated into protein by the ribosomes.
mRNA loaded LNP manufacturing
The preparation of mRNA loaded LNP is a complex process which can be split into several steps:
First, mRNA should be produced then purified before being encapsulated into the LNP.
mRNA production and purification
RNA can be produced using 3 main methods:
- Chemical synthesis: The simplest, most continuous and cost-effective method, chemical synthesis is suitable for short RNAs (<100 nucleotides) and is consequently not suitable for long mRNAs.
- Host cell: Recombinant production using a host cell (e.g Coli) still lacks scale-up manufacturing solutions despite recent scientific improvements to prevent such RNA from degradation.
- Enzymatic synthesis: Two approaches of enzymatic synthesis are nowadays unraveling the complexity of RNA synthesis: Polymerase Chain Reaction (PCR) coupled to Polymerase Chain Transcription (PCT) and advanced in vitro transcription (IVT).
As pCT still requires further improvements, especially regarding upscale manufacturing. IVT, thas naturally become the current gold standard for mRNA manufacturing. A DNA template coding for the protein of interest is transcribed into mRNA by an enzyme. The mRNA is then purified before being encapsulated into LNP.
Encapsulation into nanoparticles
Historically, lipid-based nanoparticles have always been produced in batch and by high-energy methods such as thin film hydration, high-pressure homogenization, sonication… However, those methods showed poor repeatability and yield, making them not suitable for vaccine development.
For this reason, mRNA-LNP are now manufactured using self-assembly methods: a solvent, generally ethanol in which lipids are dissolved, and an antisolvent, typically low pH water containing mRNA, are mixed.
During mixing, the 2 solutions will blend, leading to a drop in solubility of the lipids in their solvent, which triggers the self-assembly of the nanoparticles.
Considering the importance of the mixing conditions on the final nanoparticle characteristics (size, charge…) microfluidics, thanks to its unique ability to reproduce mixing conditions, as well as its ability to scale up from µL to L, is now the most used method for the manufacturing of mRNA loaded LNP.
Conclusion and future perspectives
With hundreds of mRNA-based therapeutics currently in clinical development [5], the quest for an optimal delivery system for the efficient delivery of mRNA remains a highly active domain of research for several reasons:
First, due to the instability, and large size of the mRNA, optimization of the delivery methods stays a major challenge to improve the translation from in-vitro to in-vivo, as well as improving their stability and storage, which currently requires a low temperature to avoid degradation.
Moreover, cell-specific delivery of mRNA would be beneficial for the development of mRNA therapeutics, as it would improve the delivery of mRNA to the target cell, thus reducing potential side effects while increasing efficiency doses. To this end, multiple strategies are currently being explored, such as tuning the formulation charge, or decorating NP with specific ligands to improve their specificity.
Finally, major challenges remain in scaling up the manufacturing process of those mRNA loaded LNP. In fact, currently, no platforms are available for the repeatable synthesis of nanoparticles throughout the drug development process, ranging from µL during the screening stage, to L for production.
Want to synthesize your own Lipid nanoparticle for mRNA delivery? Reach out to us!
References
- Fang, E., et al. Advances in COVID-19 mRNA vaccine development. Sig Transduct Target Ther 7, 94 (2022). https://doi.org/10.1038/s41392-022-00950-y
- Tenchov, R., et al. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement
ACS Nano 2021, 15, 11, 16982–17015 https://doi.org/10.1021/acsnano.1c04996 -
Nitika, Wei J, et al. The Delivery of mRNA Vaccines for Therapeutics. Life (Basel). 2022 Aug 17;12(8):1254. doi: 10.3390/life12081254.
-
Wadhwa, Abishek & Aljabbari, Anas & Lokras, Abhijeet & Foged, Camilla & Thakur, Aneesh. (2020). Opportunities and Challenges in the Delivery of mRNA-Based Vaccines. Pharmaceutics. 12. 102. 10.3390/pharmaceutics12020102.
- Verma M, Ozer I, Xie W, Gallagher R, Teixeira A, Choy M. The landscape for lipid-nanoparticle-based genomic medicines. Nat Rev Drug Discov. 2023 May;22(5):349-350. doi: 10.1038/d41573-023-00002-2.
