Overview of the non-viral gene delivery methods

Non-viral gene delivery methods play a pivotal role in unlocking the potential of gene therapy for addressing various genetic and acquired diseases. The ability to modulate gene expression within specific cells or tissues holds tremendous promise. Yet, major challenges remain in finding safe and efficient gene delivery vectors. This article provides a comprehensive overview of cutting-edge non viral gene delivery vectors such as liposomes, lipid nanoparticles (LNPs), polymeric nanoparticles, peptides…. We will also address their difference from viral vectors for the delivery of a wide range of gene expression modulation through exogenous nucleic acids like DNA, mRNA, siRNA, miRNA, or antisense oligonucleotides (ASO)
Introduction to gene delivery methods
The use of exogenous nucleic acid to modulate genome or RNA sequence has been investigated in clinical applications for more than 20 years. Despite their promises, their development has been hindered by technical barriers, mostly due to the lack of safe and effective delivery vectors.
Indeed, DNA and RNA are large molecules with negative charges. When introducing them into cells, various barriers affect their transfection efficiency. These include:
- Nucleases: DNA and RNA can be quickly broken down by nucleases in the blood or cells and eliminated by the body's defense systems within a few minutes.
- Cell membrane: The negatively charged DNA or RNA repels the cell's membrane, making it challenging for the gene to enter.
- Endonucleosis: Inside cells, endosomes pose a significant challenge with their multiple nucleases and low-pH environment, limiting gene transfection efficiency (generally not exceeding 4%)
- Cell structure: Microfilaments and microtubules in the cell's cytoplasm affect the movement of DNA and RNA.
- Nuclear membrane: separating the nucleus from the cytoplasm, it has small pores selective for molecules. DNA must rely on the rupture of the nuclear membrane during cell division to enter the nucleus for transcription.
Overcoming these barriers requires safe and efficient gene delivery systems, making them a key element for gene therapies.
To cross those barriers viral vectors have been widely used in clinical trial (around 70% of the clinical trials carried out with them) to deliver oligonucleotides. While they helped advance the field, they also came with major limitations, including carcinogenesis, immunogenicity, and limited DNA packaging capacity.
To overcome those constraints, researchers have combined material sciences, nanotechnology, and nucleic acid chemistry to develop novel non-viral gene therapy, addressing safety concerns, lower immunogenicity, and the ability to deliver larger genetic payloads.
Viral vs non-viral vectors
Both viruses and non-viral vectors have been used to deliver drugs or genetic material to cells. However, they have different characteristics and applications.
Viral vectors
Viral vectors are derived from viruses, which are infectious agents that can infect and replicate within cells. There exist 5 main classes of viral vectors, that can be grouped in two categories can be grouped into two categories based on whether their genomes integrate into the host cellular chromatin (oncoretroviruses and LV) or persist mainly in the cell nucleus as extrachromosomal episomes (AAV, AV, and herpes viruses). [2] All of them can effectively deliver genetic material to cells because they have evolved to hijack the cell's machinery for replication. This can lead to high transfection efficiency, which means that a large number of cells can be infected with the vector. However, viral vectors tend to cause immune reactions, leading to major side effects detrimental to the success of clinical trials, and integration of the viral DNA into the host genome, which can lead to long-term side effects. In addition to this, viral vectors offer a limited packaging capability (generally from 5 to 8kb), making them unsuitable for long RNA strands (such as mRNA)
Non-viral vectors
Non viral vectors are synthetic particles or molecules that are not derived from viruses. They are a broad category as they combine both naked DNA/RNA, as well as more specifically engineered structures such as lipid-based or polymeric nanoparticles (Liposomes, LNP, PLGA…)
Non-viral vectors cannot replicate within cells, so they must rely on other mechanisms to deliver their cargo. This can lead to lower transfection efficiency than viral vectors - though the novel generation of nonviral vectors such as Lipid nanoparticles (LNP) mostly overcome this limitation - but it also means that non-viral vectors are less likely to cause immune reactions or integrate their DNA into the host genome and that they can package larger DNA and RNA strand, making them a more flexible tool overall.
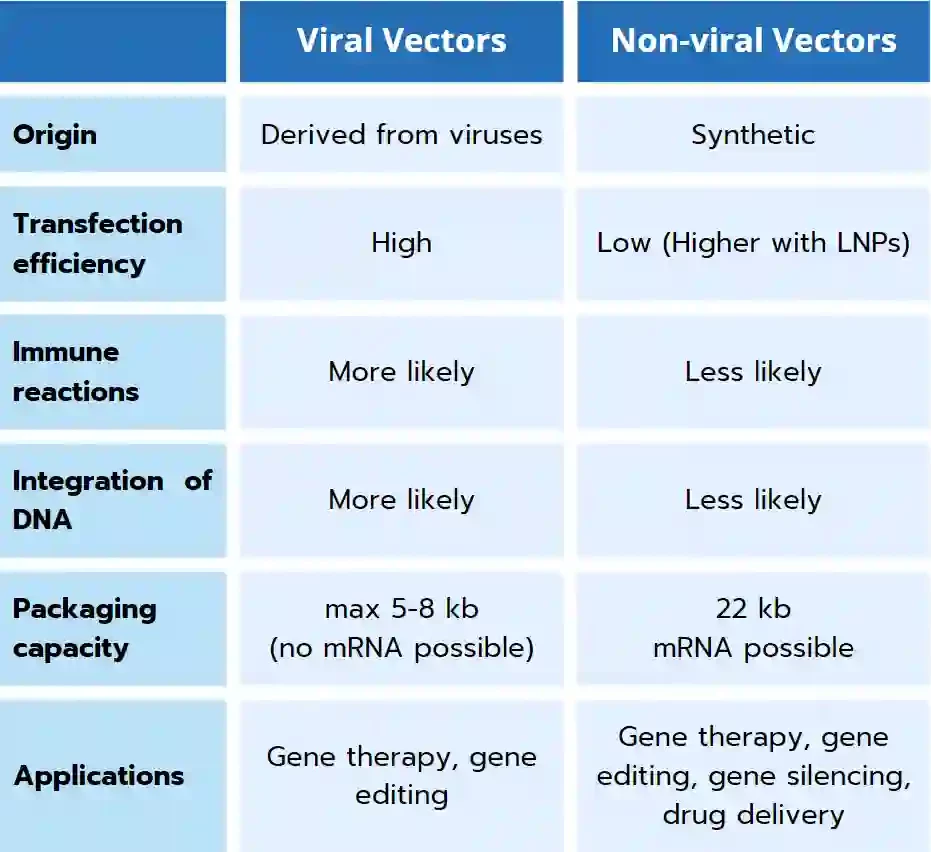
Therefore, the choice of whether to use a viral or non-viral vector as a gene delivery system depends on the target application. Viral vectors are often used when high transfection efficiency is required. Instead, as they offer a safer alternative, non-viral vectors are often used when immune reactions or integration of DNA are a concern, such as in gene therapies, gene silencing, vaccines, or drug delivery.
Key characteristics of the Non-viral gene delivery vectors
As we discussed above, successfully introducing genes into cells faces challenges due to the negative charge oligonucleotides, making it difficult to cross the cell membrane. To simplify their delivery non-viral gene delivery system are used. However, those do not carry ideal properties and thus have to be specifically engineered for optimal transfer efficiency, specificity, gene expression, and lower toxicity. Most critical parameters include composition, size, and charge as they greatly impact their stability, toxicity, and cellular uptake.
Composition
Due to the negative charge of the RNA and DNA, cationic vectors generally play a vital role in encapsulating genes into nanoparticles through electrostatic or hydrophobic interactions. However, when circulating through the body, highly positively charged particles become very toxic, due to the adsorption of particles at their surface. A trade-off between the use of positively charged lipids/polymers or not should be found.
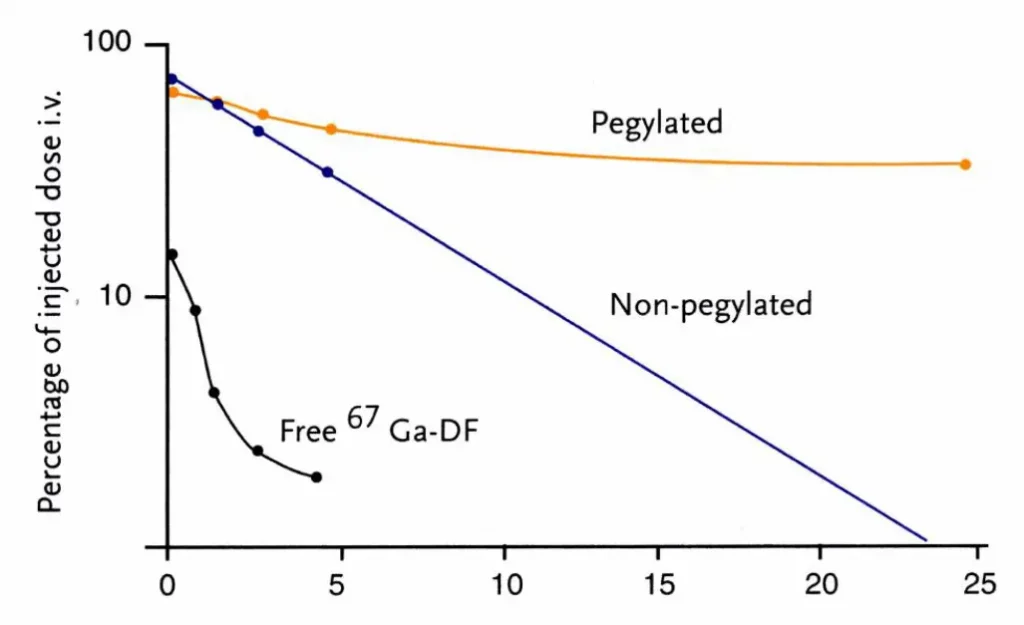
In addition to the overall charge, the presence of stealth agent, such as a polyethylene glycol (PEG), helps diminish the interaction between dendritic-structured polymer micelles and serum proteins, leading to a substantial enhancement in the serum stability and circulation time.
Additionally, the ratio of delivery vehicle material to oligonucleotide plays a major role in the transcription efficiency efficiency.
Size
Selecting the right nanoparticle size is key, as it greatly impacts their delivery efficiency and toxicity/side effects.
Indeed endocytosis of lipid-based or polymeric nanoparticles, depending on their size, follows three pathways:
- Phagocytosis: Primarily used to eliminate foreign particles, thus not ideal for gene uptake.
- Microcytocytosis: Involves larger particles ranging from 200 nm to 5 μm in size
- Receptor-mediated endocytosis: Currently the most common pathway, it is functional for nanoparticles with size up to 200 nm. This pathway has three subtypes—clathrin-mediated endocytosis (CME), clathrin-independent endocytosis (CIE), and macropinocytosis.
In addition to this, nanoparticles smaller than 50 nm are generally quickly cleared by the kidneys, while nanoparticles larger than 300 nm tend to activate the body’s immune response.
For all these reasons, nanoparticle sizes in the order of 60 to 100 nm are generally used as they prove to be more easily endocytosed while being easily expelled by cells through cytosolic excretion, therefore making them less toxic. Learn more on our review on the influence of nanoparticle size.
Surface
Surface charge
Surface charge matters as well. Neutral or positively charged nanoparticles (with a positive Zeta potential) are best suited for cellular uptake, due to the negative charge on the cellular membrane surface. On the other hand, too high charges can lead to toxicity, while too low leads to an unstable nanoparticle. A clear balance of charge is therefore crucial to ensure lowered cytotoxicity, better stability, and improved cellular update. Therefore, nanoparticle with low Zeta potential (35 mV) are typically used.
Surface functionalization & targeting strategies
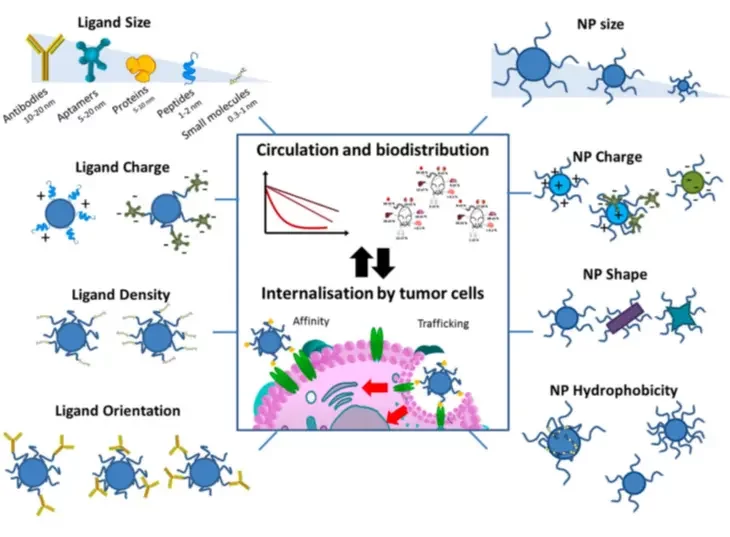
To improve cellular uptake and specificity, nonviral vectors can also be functionalized using specific ligands or molecules like antibodies, glucose, transferrin, folic acid, transporter proteins, and integrin ligands, to better bind to the target cell. Learn more about nanoparticle targeting strategies in our review.
Other factors
Multiple other factors can also impact the final in-vivo transcription efficiency. For instance, overly tight DNA packing can hinder transcription. Also, nanoparticles shape also has an effect: for instance, nanoparticles possessing a rod-shaped geometry tend to exhibit superior cellular internalization efficiency. Additionnaly, the requirements of the delivery vehicle will change depending on the payload (DNA, siRNA, miRNA, ASO, mRNA…) both due to the different target locations within the cells and their size. [3] Finally, the payload itself can be modified to be better internalized and delivered to the cells. [4]
Non-viral vectors for gene delivery
The non-viral vector field is a fast-paced research area marked by constant advancements. However, in the past few years, three main classes of non-viral vectors have emerged as the most promising: Polymers, lipids and peptides
Lipid-based vectors
Lipids have been the main go-to solution for gene delivery since the 1980s, as they offer excellent biocompatibility, lowered toxicity, and they can encapsulate both hydrophilic and hydrophobic payloads.
The first commercially available siRNA therapy (Onpattro) was based on a lipid-based vector: a lipid nanoparticle or LNP - very similar to ones used in the mRNA COVID-19 vaccines.
Liposomes
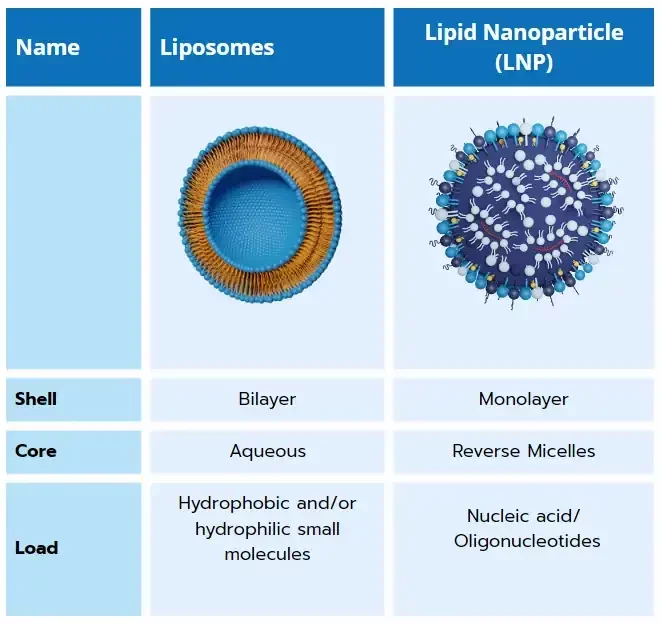
Liposomes, first discovered in the 60s, are vesicles with a lipid bilayer, that can encapsulate both small and large molecules, including RNA or DNA.
Their most simple structure consists of phospholipids with hydrophilic, polar head groups and hydrophobic, non-polar tails, assembling in a bilayer. They can be further functionalized by incorporating PEG (polyethylene glycol) in their formulation to improve the stealth effect, and thus circulation time. While functional, their simple neutral structure does not allow for high encapsulation efficiency or efficient delivery to cells.
Lipid nanoparticles (LNP)
Lipid nanoparticles are the cornerstone of the ongoing RNA-LNP/gene delivery revolution. They are a key component of the 3 currently approved RNA therapies (Onpattro & the 2 covid vaccines), and show great promises with more than 300 ongoing trials!
LNP emerged to overcome the primary limitation of liposome technology: its poor interaction with nucleic acids due to their neutral charge. To address this, cationic lipids were initially used to enhance interaction with negatively charged RNA, improving encapsulation efficiency. However, regular cationic lipids increased toxicity by triggering immune responses and cytotoxicity, rendering them impractical.
To overcome this challenge, a novel type of lipid, known as ionizable lipids, was developed. These lipids can change their charge with pH, becoming positively charged at low pH (facilitating RNA encapsulation, and RNA ) and remaining neutral at physiological pH, ensuring better biocompatibility during drug injection into the body.
In addition to maximize encapsulation efficiency - reaching up to 95% when using microfluidic-based methods to formulate them - the use of ionizable cationic lipids improves delivery. Upon entering the endosome, where pH decreases, lipids regain a positive charge, destabilizing the particle and facilitating nucleic acid delivery to the cell.
Combining ionizable lipids with PEG, cholesterol, and a helper lipid creates a new type of lipid-based nanoparticle with a unique structure. These lipid nanoparticles (LNPs) consist of a phospholipid, PEG, and ionizable lipid layer surrounding a core that entraps nucleic acids. By adjusting the composition, including the nature and ratios of each component, one can finely control the interaction with the entrapped nucleic acid, tuning encapsulation efficiency and zeta potential. It has for instance been demonstrated that picking the right ionizable lipid, can improve the final in-vivo transcription efficiency by a factor of up to 100!
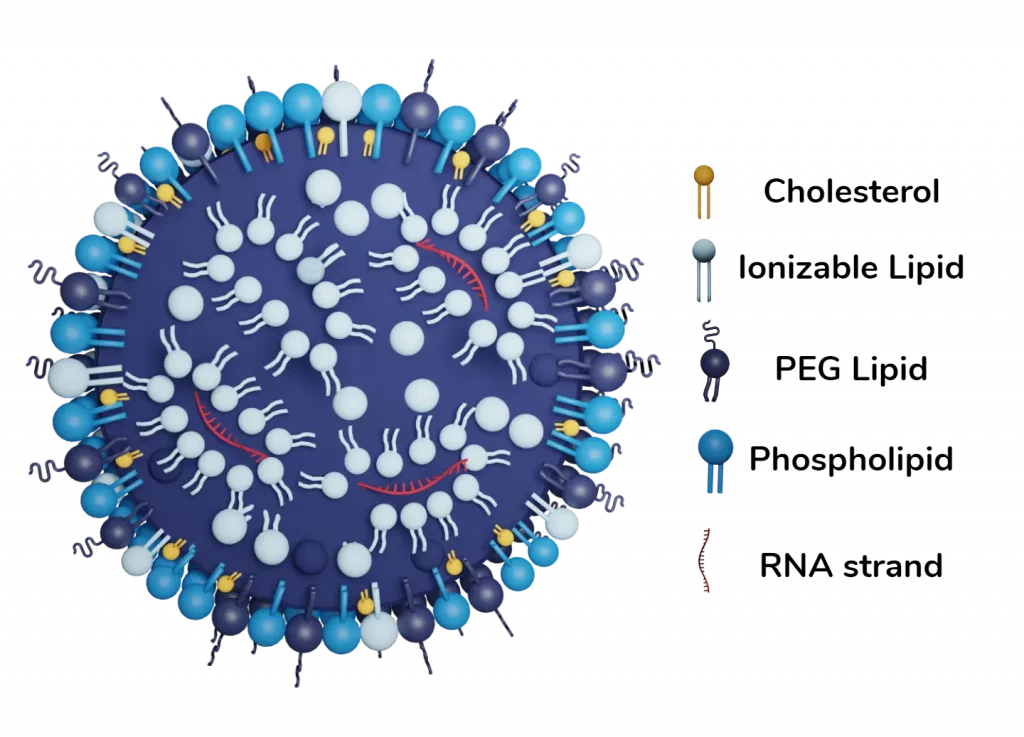
Yet, because nobody is perfect, LNPs still have several drawbacks that the ongoing research is seeking to overcome: First, commercially available RNA-LNP solution requires very low (-80°C) storage temperature, though it has been improved in the novel generation of vaccines. Additionally, the PEG composing the shell membrane can lead to allergies, and thus side effects on patients. Developping novel PEG-free LNP formulation has thus become a major challenge in the community. Finally, LNP tend to agglomerate into the liver, therefore passive or active targeting is required to access other organs.
Want to synthesize nanoparticles using microfluidics?
Discover our innovative and user-friendly nanoparticle synthesis platforms
Lipidoids
Lipidoids are materials similar to lipids, created by combining amines with lipophilic acrylates, acrylamides, or epoxides. They are gaining attention due to their solvent- and catalyst-free synthesis, enabling rapid screening of various lipidoid structures. Wide librairies of lipidoids have been synthesized, showing effective gene transfer and expression in mice, rats, and nonhuman primates. Recently, Molla et al. formulated 13 lipidoids as liposomes to deliver siRNA [6] demonstrating superior knock-down efficiency compared to commercial reagents. These findings suggest potential exploration of lipidoids for gene delivery.
Polymers
Polyethylenimine (PEI)
In 1995, scientists created Polyethylenimine (PEI), the first polycationic polymer used in gene therapy. It has special amine groups arranged on its chain, partially protonated at normal pH but fully protonated in acidic endosomes. PEI's charged nature induces an osmotic effect("proton sponge effect"), causing endosomes to burst and enhance gene delivery. Although PEI is the standard for measuring transfection efficiency, it has drawbacks, like lack of specificity and non-biodegradability, causing cytotoxicity. This proves especially critical in the context of gene therapies, as multiple expositions are usually required.
Researchers have made progress in understanding these limitations. Studies explored how PEI interacts with endosomal lipids and escapes late endosomes during delivery. This understanding is crucial for designing effective delivery vector.
Modified PEI
The primary challenge faced by PEI vectors is their cytotoxicity, attributed to non-degradable amide bonds, leading to accumulation and disruption of metabolic activity in normal cells. The degree of cytotoxicity is positively correlated with the molecular weight of the polymer. A promising approach to mitigate toxicity involves employing ligand-modified low molecular weight PEIs, such as those incorporating folic acid or various functional peptides. Studies demonstrated that self-assembled nanoparticles (SNPs) created from cyclodextrin-grafted low molecular weight PEI exhibited low cytotoxicity and higher delivery efficiency than regular PEI.
An alternative strategy to maintain PEI biocompatibility and reduce toxicity involves combining poly lactic-co-glycolic acid (PLGA) nanoparticles with arginine-modified PEI polymers (AnPn). The addition of AnPn significantly improved the nuclear localization of pDNA and successful gene expression in primary human astrocytes.
Biodegradable polymers
Repeated administration is often required for gene therapy, and to minimize cytotoxicity, biodegradable polymeric vectors, both synthetic and natural, are preferred over non-biodegradable ones. Synthetic polymers offer versatility and consistency but may lack cell interaction, while natural polymers, like chitosan, are biocompatible but face batch variation issues.
Chitosan
Natural polysaccharides are highly biodegradable, biocompatible, and non-toxic. It forms complexes with genetic material in acidic conditions and can be modified to enhance cellular entry and specificity. Chitosan-coated nanoparticles are actively studied for brain cancer gene therapy.
Poly(β-amino ester)s (PBAEs)
They show robust transfection capabilities and efficient endosomal escape. Hyperbranched PBAEs with cationic and anionic charges offer improved capabilities. Highly branched PBAEs with biodegradable units achieve higher efficiency.
Polylactide (PLA)
PLA is a synthetic biodegradable polymer, is extensively used in drug delivery. Modified cationic polylactide is suitable for gene therapy. Aminoglycosides, and antibiotics, have been explored as new cationic polymeric vectors for gene transfer in 2020. The full potential of polymer-based delivery systems is still to be fully realized.
Peptides
Peptides are short chains of 2–50 amino acids that can be designed for self-assembling nanoscale structures. They interact with genetic material to form peptiplexes, facilitating delivery either through conjugation or electrostatic forces. Numerous types of peptides can be used depending on the cargo and target cell, including:
Peptide nucleic acid (PNA) conjugates. They consist of stable, uncharged molecules linking peptide and nucleic acid via covalent bonds.
Polypeptides, like poly(L-lysine) (PLL): They are widely used for gene delivery due to their high charge density allowing effective DNA condensation. However, PLL has limitations in transfection efficiency and endosomal escape. Modifications, such as combining PLL with other peptides, can help enhance endosomal escape.
Polypeptides can be designed into dendrimers, providing positively charged groups for genetic material complexing, cellular membrane passage, and buffering capacity for endosomal escape. Recent studies have diversified amino acids in dendrimers, changing flexibility and charge distribution, leading to increased cellular uptake. Dendrimers can incorporate lipids or polymers for enhanced efficacy, as demonstrated by improved transfection with added polyol to lipid/dendrimer hybrids or polymer excipients.
Conclusions
Non-viral gene delivery is a promising field with the potential to revolutionize the treatment of a wide range of diseases. This article has provided a comprehensive overview of the latest advancements in non-viral gene delivery, including liposomes, lipid nanoparticles (LNP), polymeric nanoparticles, and peptides. These vectors offer several advantages over viral vectors, including lower toxicity, reduced immunogenicity, and the ability to deliver larger genetic payloads. Additionally, non-viral vectors are well-suited for gene therapy applications, as they can be specifically engineered to target specific tissues or cells.
As research in this field continues to progress, we can expect to see even more innovative non-viral gene delivery vectors emerge. These vectors will have the potential to treat a wider range of diseases and improve the efficacy of gene therapy.
Want to discuss your choice of non viral delivery method?
Speak to our application scientist
References
[1] Bertrand, Nicolas et al. “Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology.” Advanced drug delivery reviews vol. 66 (2014): 2-25. doi:10.1016/j.addr.2013.11.009
[2] https://www.liebertpub.com/doi/full/10.1177/1535676019899502
[3] Wang, C., Pan, C., Yong, H. et al. Emerging non-viral vectors for gene delivery. J Nanobiotechnol 21, 272 (2023). https://doi.org/10.1186/s12951-023-02044-5
[4] Roberts, T.C., Langer, R. & Wood, M.J.A. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov 19, 673–694 (2020). https://doi.org/10.1038/s41573-020-0075-7
[5] Zu, Hui, and Danchen Gao. “Non-viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects.” The AAPS journal vol. 23,4 78. 2 Jun. 2021, doi:10.1208/s12248-021-00608-7
[6] https://pubs.acs.org/doi/10.1021/acs.bioconjchem.0c00013
NANOPARTICLE KNOWLEDGE
Looking to learn more about nanoparticle ? Do you need inspiration or a different angle to take on your specific problem? Well, we probably have an application note just for you, feel free to check them out!
Endosomal Escape: A Critical Challenge in LNP-Mediated Therapeutics
Endosomal escape of lipid nanoparticles (LNPs) is a crucial process in the intracellular delivery of mRNA-based therapies and vaccines. After cellular uptake via endocytosis, LNPs must efficiently release their mRNA payload from endosomes into the cytoplasm to ensure proper translation into therapeutic proteins. This step is vital for the success of LNP-mediated delivery systems, as…
Introduction to PLGA Nanoparticles as a Drug Delivery System
For the past decades, modern medicine shifted more and more towards the use of nanocarriers as drug delivery systems (DDS) to overcome the inherent drawbacks of traditional drug administration. To that end, several drug carriers were developed, ranging from lipid nanoparticles (LNPs), and solid nanoparticles (SLNs), to polymersomes, liposomes, and polymeric nanoparticles. On the latter,…
The Evolution of Nanomedicine: from ideas to clinical applications
Nanomedicine represents a transformative approach to diagnostics and therapeutics, leveraging the unique properties of materials at the nanoscale. Its roots extend back to the mid-20th century when the concept of using nanometer-sized particles for medical applications began to materialize. The field has grown significantly over the decades, offering novel solutions in drug delivery, imaging, and…
