A complete guide to understanding Lipid nanoparticles (LNP)

Introduction to lipid nanoparticles
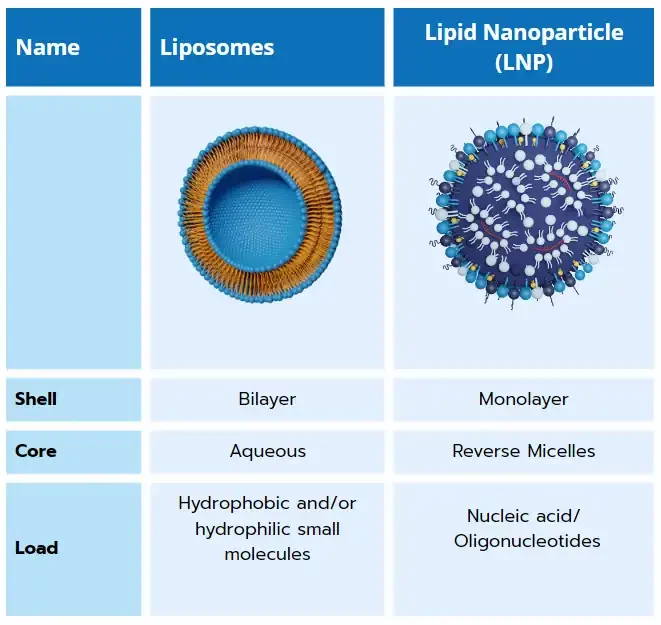
Resulting of 50 years of research in the nanocarrier delivery field, lipid nanoparticles are close cousins to other lipid-based nanoparticles such as liposome and solid lipid nanoparticle (SLN). Unlike those which are specialized in the encapsulation of hydrophobic and/or hydrophilic small molecules, LNP have the unique capability of encapsulating nucleic acids such as messenger RNA (mRNA), small interfering RNA (siRNA), transfer RNA (tRNA)…. And deliver them to a specific location in the body.
Composed of a lipid shell surrounding an internal core made of reverse micelles that encapsulate oligonucleotides, those non-viral delivery systems offer both protection from enzymatic degradation to their cargo and efficient RNA delivery to the target cells.
Those unique features open up immense possibilities for researchers who wish to develop their ribonucleic-based therapeutics and accurately deliver them to a specific location in the body. This groundbreaking technology was brought to the spotlight by BioNtech and Moderna, through the Covid 19 mRNA-LNP vaccine they provided.
Throughout this article, we will give a detailed introduction to :
- Why using lipid nanoparticles
- Their main characteristics ( composition and structure)
- How to fabricate them
- How to characterize them
- The delivery process to the cells
- Typical application
What makes lipid LNP unique?
While other lipid-based nanoparticles can be used as a carrier for a wide range of small molecules, their simple structure poses an issue for the generally negatively charged nucleic acids, such as mRNA and siRNA, for several reasons:
1/ The weak interaction between the usually neutral lipids and negatively charged oligonucleotides complicates its encapsulation and intracellular delivery.
2/ When introduced to the body, other types of liposomal carriers are subject to clearance from the reticuloendothelial system (RES), leading to a decrease in circulation time in the body.
From liposomes to LNPs
As its offspring, lipid nanoparticles exhibit very similar characteristics to liposomes, though with one major change making it unique: the use of a ionizable lipid.
One main limitation of the current liposomal carriers is the poor interaction with nucleic acids due to their neutral change. For this reason, cationic lipids were used to help with the interaction of negatively charged RNA, leading to improved encapsulation efficiency. However, the use of regular cationic lipids also leads to an increased toxicity by triggering an immune response and cytotoxicity, thus not being a viable solution.
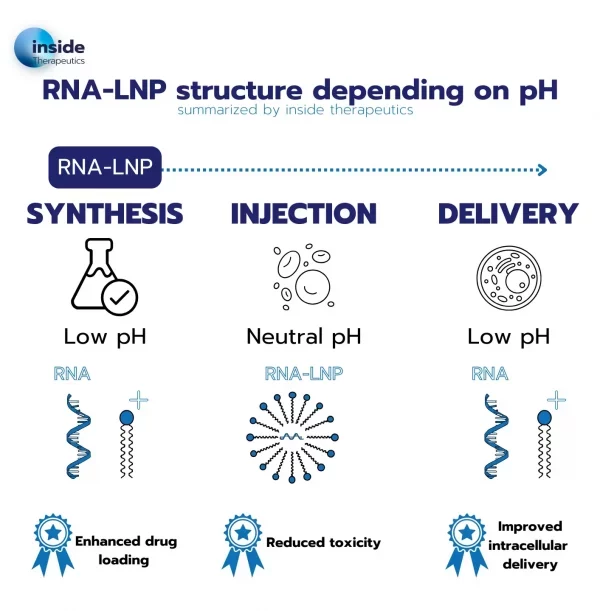
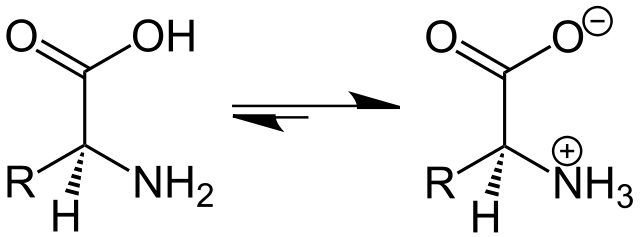
To tackle this issue, a new kind of lipid known as ionizable lipids was engineered. Those ionizable lipids have the unique capability of switching charge with the pH and being positively charged at low pH while remaining neutral at physiological pH.
This unique capability permits an easy encapsulation of the RNA at low pH while offering much better biocompatibility when brought back to physiological pH for the drug injection into the body.
In addition to better encapsulation, according to the membrane phase transition theory, the use of the ionizable cationic lipid also allows for improved endosomal escape: upon entering the endosome, where the pH value drops, lipids get charged again, leading to a destabilization of the particle and thus an easier delivery of the nucleic acid to the cell.
Finally, the combination of the ionizable lipid with PEG, cholesterol, and a helper lipid allowed for the generation of a whole new kind of lipid-based nanoparticle with a unique structure and morphology. LNP are formed by a phospholipid, PEG and ionizable lipid layer surrounding a core in which ionizable lipids are entrapping the nucleic acids.
By adjusting the nature and ratios of each component, one can then fully regulate its interaction with the entrapped nucleic acid and thus tune the encapsulation efficiency, zeta potential… Making the balance of the composition a key challenge of the drug development.
Lipid nanoparticles formulation
What is true for your diet also happens to be true at the nanoscale, where there is both good and bad fat for your body. In the recently developed RNA vaccines, the interactions of the body with the different lipids contained in the nanoparticle are the main reason for the unwanted side effect such as aches, inflammation…
Despite their already much-improved biocompatibility compared to other lipid-based nanoparticles thanks to the use of ionizable lipids, major challenges remain to reach a better control of their physiochemical properties, all along the drug manufacturing scale-up.
Therefore, the choice of lipids is crucial to both optimize the LNP encapsulation capability and the delivery of the payload to the target cells.
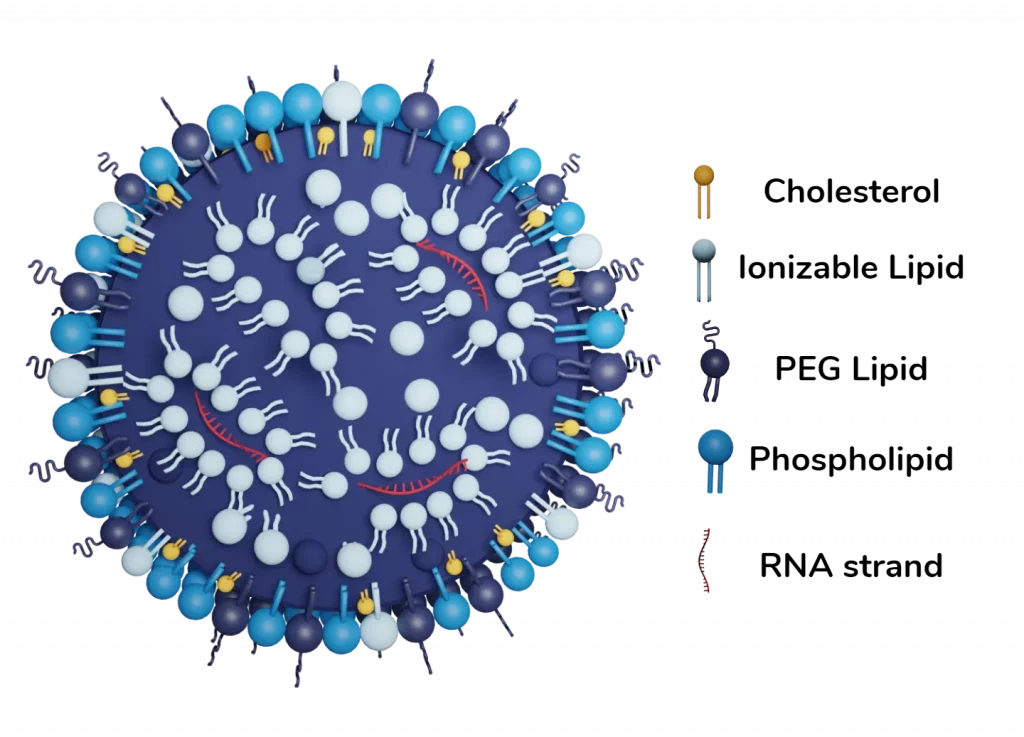
All the current FDA-approved formulations are composed of 4 lipids (glycerophospholipids, cationic lipids, sterol lipids, and PEGylated lipids) each having a very specific role to encapsulate the oligonucleotides into aqueous phase. In addition to this, a selection of surfactants can be used to tailor their properties.
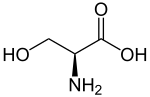
Ionizable cationic lipid
Neutral at 7 pH, they switch to positively charged below their acid-dissociation constant (pKa). They serve 3 functions: when positively charged:
1/ They allow for a more efficient encapsulation of the RNA during the fabrication.
2/ They ensure better nucleic acid delivery in the cytosol.
3/ When neutral at neutral pH, they permit lowered nanoparticle toxicity for the body.
Ionizable lipids are the highest proportion of all the lipids present in the LNP with typically 50 mol%, as they are needed to both bind and encapsulation the oligonucleotides cargos. They are the main component of the inner core and serve to trap the nucleic acid inside vesicles. They can also take part in the membranes, in which when they change of charge in the cytosol leads to its structural disruption of the LNP and the cargo delivery to the cell.
Pegilated Lipid (PEG)
Despite being the smallest %age of all the lipids composing the LNP, the pegylated lipid has a major impact on several key characteristics including size, aggregation, stability, and encapsulation efficiency… All these properties are related to both the molar ratio of the PEG, and the length of the lipid tail and PEG chain.
Studies show that even in small concentrations PEG highly helps with the size control of the LNP population, which can increase to 200 nm with 0% PEG, instead of 100nm with as low as 0.5 mol%.
PEG also appears to help prolong circulation time by minimizing liver uptake and therefore helps increase the overall drug delivery system efficiency.
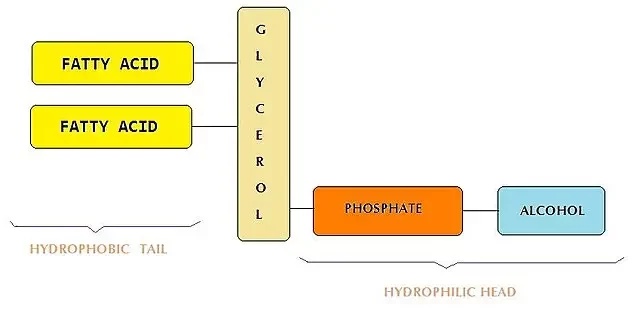
Phospholipids
Phospholipids were the major components of the original lipid-based nanoparticles from which the LNP is derived and that contained nearly 85 mol% of it. Since then, its role and %mol has decreased in favor of the ionizable lipids. The current commercial LNP formulation contains around 10% of phospholipids, generally in the form of DSPC. Mostly present in the membrane, phospholipids mostly help improve encapsulating efficiency.
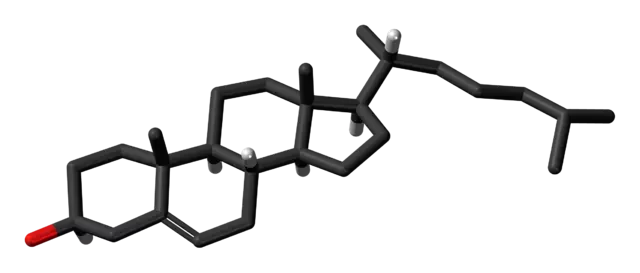
Sterol lipids
The sterol lipids – generally cholesterol - are helper lipids, and their role mostly depends on the compound they are associated with. When combined with phospholipids helps with the rigidity and stability of the membrane and pushes the lipids toward a liquid-ordered phase. Thanks to this, sterols facilitate stable encapsulation, help decrease drug leakage, and greatly improve drug stability over time.
Lipid nanoparticles Synthesis methods
A wide varieties of methods are available for the synthesis of LNPs, each offering a different trade-off in terms of the characteristics of the generated LNPs, encapsulation efficiency and scale-up capabilities.
Three main categories of methods can be defined: The high energies methods, the low energy methods, and the solvent-based methods.
The pros and cons of each method are summarized in the below table, and more details about them can be found in our LNP manufacturing review.
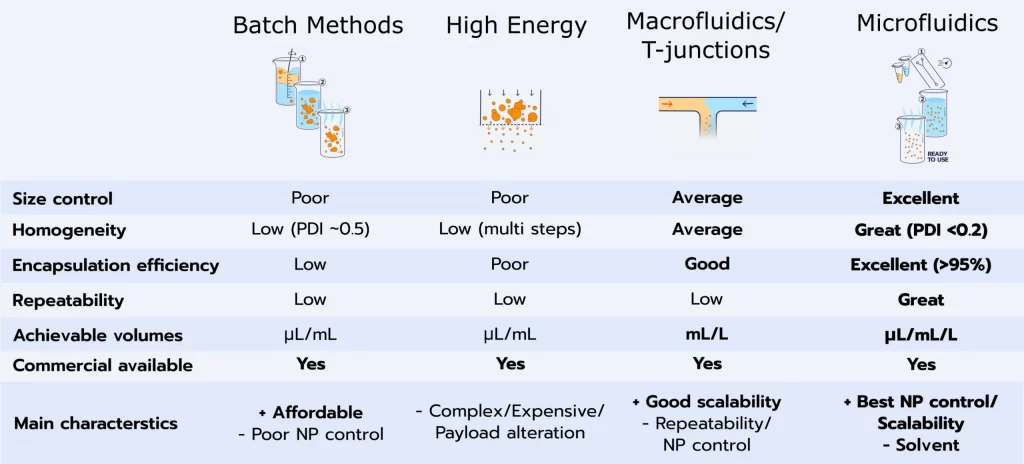
Batch methods
Batch methods such as thin film hydration methods are solvent based methods where a solvent containing the lipid mix is vaporated. The remaining lipids are then mixed together with an antisolvent such as water containing the RNA, triggering nanoparticle assembly. They are relatively easy to use yet offer near no size control,homogenization poor encapsulation efficiency, and can hardly be scaled.
High energy
High-energy methods such as high-pressure homogeneization and sonication are top to bottom approach using the high energy provided by the system to break down the particles into smaller ones. Their main advantage is to already be available and easily scalable. They can be used either alone or in combination with other methods. However, they provide very poor control of the generated NP and show very low encapsulation efficiency. The use of high energy also leads to high risks in terms of lipids and drug alteration during their synthesis.
Macrofluidics/T junctions
Unlike the 2 above methods, macrofluidics approach are bottom-up methods. In those solvent-based processes, a solvent containing the lipids (usually ethanol) is mixed with an antisolvent (usually water) triggering the lipid nanoparticles nanoprecipitation. (see our review on nanoparticle self-assembly mechanism for more).
To control the mixing condition, T-mixers, such as impingement jet mixers, have been used. While they are highly effective for large-scale production, such as in the manufacturing of the COVID-19 vaccine, these methods necessitate the use of substantial liquid volumes, making them unsuitable for early-stage drug delivery processes (screening ot in-vivo). Moreover, they inherit some of the disadvantages associated with high-energy techniques, mainly due to the elevated pressures involved, which can potentially lead to the degradation of the RNA compounds.
Note that a purification step is required following synthesis to ensure solvent removal, hence lowered toxicity and better stability.
Microfluidics
To overcome the main limitation of macrofluidics methods, LNP microfluidics manufacturing methods have been developed.
Thanks to its unique repeatability and capability to perfectly control mixing conditions, microfluidics permits the highest level of control and repeatability in terms of lipid nanoparticle manufacturing.
On top of that, the possibility to handle very small volumes with microfluidics at low pressure has made it the gold standard for LNP manufacturing all along the RNA therapies or vaccine development process. Learn more in our microfluidics synthesis methods review.
Typical performance achievable with microfluidics are size in the 50 to 200 nm range while being highly controllable, repeatable and homogeneous (PID <0.2). Encapsulation efficiency usually reaches 90% or above with such methods.
Looking to synthesize nanoparticles using microfluidics?
Discover our innovative and user-friendly nanoparticle synthesis platforms
Lipid nanoparticle characterization
As described above, the physiochemical attributes of LNPs need to be precisely controlled throughout their synthesis to reach the targeted area and efficiently deliver their payload to the cells.
While there is currently no regulatory framework for nanomedicines a set of general characterization guidelines is available.
The most important physiochemical parameters are: LNP size and size distribution (characterized by the polydispersity index or PDI), morphology as well as their Zeta potential. Drug loading and encapsulation efficiency are also important parameters to be considered to evaluate the overall LNP encapsulation effectivness.
In addition to these, drug makers in the pharmaceutical industry should also investigate biodistribution all throughout their clinical trials to ensure medicine’s efficiency.
Size and distribution
LNP size is a crucial factor that impacts its quality, including stability, encapsulation efficiency, biodistribution and cellular uptakes.
Optimal nanoparticle size varies based on payload, target cells and experimental models, and should thus be optimize for each drug.
Small nanoparticles offer the advantage of easily penetrating the cells through endocytosis while avoiding kidney and liver clearance. Nevertheless, when too small, the cells tend to have difficulties expelling them, leading to toxicity.
Therefore, the optimal LNP size varies depending on the target organ and application. A general compromise is to use a size of around 60-100 nm for cell uptake, while LNP in the 50 - 60nm range is preferred for tumor extravasation.
The Polydispersity Index (PDI) describes the non-uniformity of the nanoparticle population, influencing both drug delivery efficiency and toxicity. It is a dimensionless parameter, defined as the square of the LNP population Standard deviation/average value. Therefore, it varies from 0 to 1, 0 being homogeneous, and 1 being heterogeneous.
The FDA guidelines consider polydispersity as a key element to characterize for organic nanoparticle, and the industry generally suggests using a PDI <0.3, as this value indicated an homogeneous particle population . Using advanced microfluidics technology permits to achieve an optimal PDI within a formulation, typically around 0.1 for RNA-LNP.
Interestingly, it should be highlighted that the size of lipid nanoparticles is primarily determined by the lipid composition and synthesis conditions, rather than the size of the RNA cargo
Size and PDI characterizations are generally achieved using optical methods such as dynamic light scattering (DLS), NTA (Nanoparticle tracking analysis) or laser diffraction (LD).
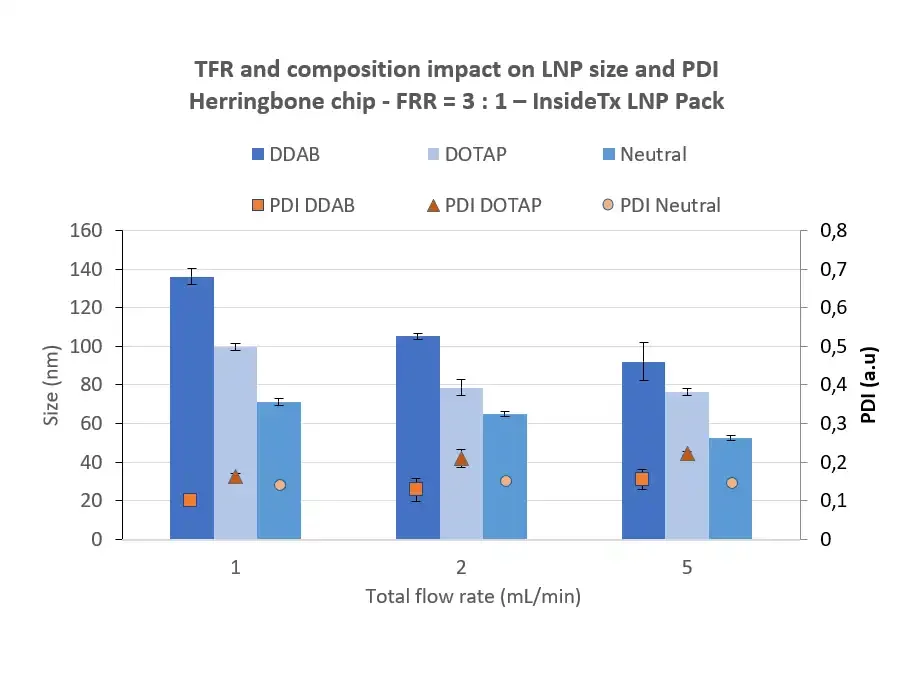
Zeta potential
The Zeta potential is a measure of the magnitude of the electrostatic field close to the particle's surface.
A too-neutral LNP (low Zeta potential) will lead to an increased aggregation during manufacturing, as the charge will not repel each other. On the other hand, a too-high charge at the surface will lead to toxicity as described previously. For this reason, a zeta potential in the order of -30 mV is generally considered optimal.
Zeta potential is characterized using a Zeta Potential Analyzer
Surface morphology
Microscopy methods such as Transmission electron microscopy (TEM or Cryo-TEM) or Atomic Force Microscopy (AFM) are generally used to characterize the lipid nanoparticle morphology, each offering a different compromise. AFM allows for a 3D mapping of the particle, while TEM offers a view of the internal composition of the LNP.
LNP Load capacity (DL) and Encapsulation Efficiency (EE%)
Load capacity and encapsulation efficiency characterize the LNP’s ability to efficiently encapsulate the oligonucleotide.
The Load capacity corresponds to the percentage of trapped API over the total LNP weight. Instead, the encapsulation efficiency measures the percentage of RNA that has been successfully encapsulated (i.e trapped nucleotides over the initial amount in the formulation).
The most effective synthesis methods – such as microfluidics - manages to reach an encapsulation efficiency above 90% for mRNA or siRNA. (check our Userguide for more applicative data)
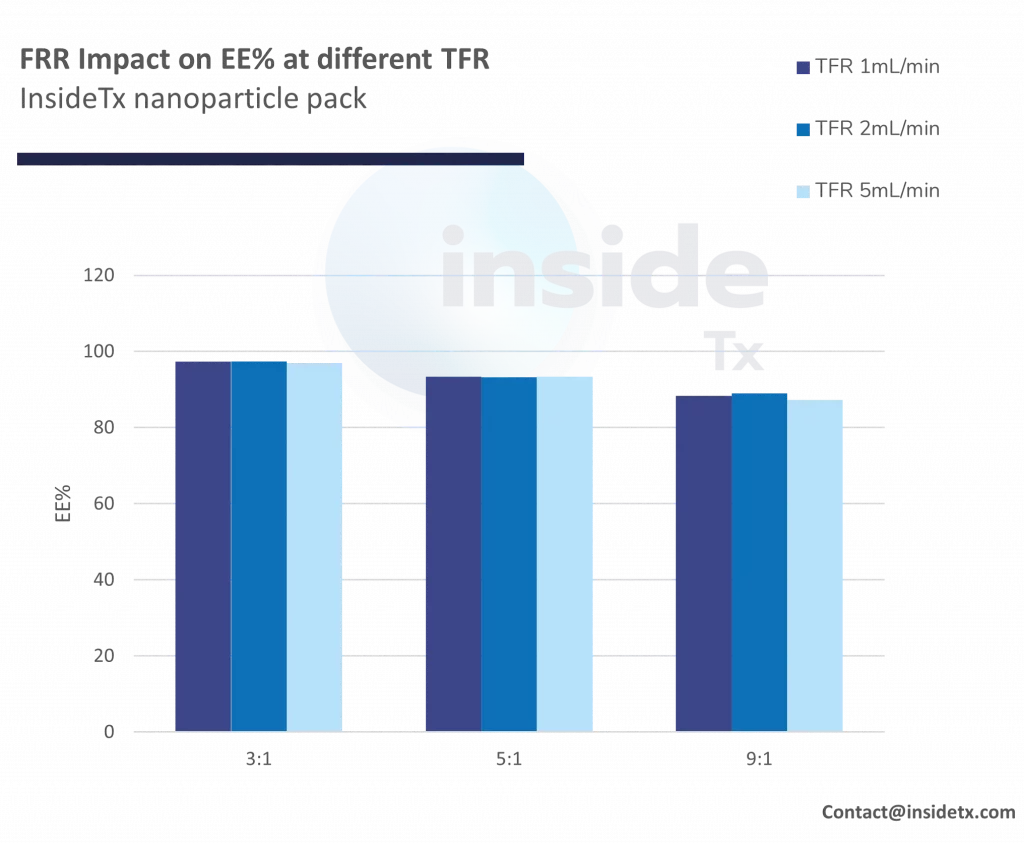
Lipid nanoparticles delivery mechanism
When introduced to the body, RNA and small molecules are overall unstable and prone to degradation by the serum endonucleases.
Historically, gene and cancer therapies were delivered using viral formulations, where viruses are used to deliver nucleic acids to the cells. While mastered, those methods are not optimal as they still show multiple side effects (strong immune responses, hepatotoxicity...) and only have a small loading capacity (~5kilobases).
As described in the review on lipid-based nanoparticles, in the past 50 years liposomes and other types of lipid-based nanoparticles have extensively been used for drug and small particle delivery. However, their simpler structure does not allow for efficient nucleic acid encapsulation and delivery to their target cell.
For all these reasons, the development of LNP optimized for oligonucleotides’ transport opened new possibilities for the treatment of a wide range of diseases through the delivery of genetic material to the cells.
A great variety of payloads can be efficiently encapsulated and delivered to the target cells using LNP. They not only allow for the delivery of nucleic acid therapeutics, such as messenger RNA (mRNA), small interfering RNA (siRNA), and small activating RNA (saRNA) but also small drugs (for cancer treatement for instance), peptides, proteins, as well as ribonucleoproteins such as Cas9.
RNA, DNA and small molecules delivery
The initial steps of the intracellular delivery mechanism of RNA and small molecules/drugs are relatively similar: The improved circulation time of the LNP allows them to enhance their targeted delivery to specific tissues, where they extravasate and accumulate into the interstitial fluid.
At that stage, if the LNP contains small molecules/drugs, it will simply release the drug over time, which will diffuse across the target cell. If they are targeted via ligands against the cell, LNPs can cross the target cell membrane and lead to the release of the drug within the cell itself.
When containing nucleic acid, LNP’s attachment against the cell surface receptor leads to an endocytotic uptake. The decrease of pH during the maturation process of the endosome leads to a change of charge of the ionizable lipid, destabilizing the LNP structure which triggers the endosomal escape, releasing the nucleic acid into the cytoplasm.
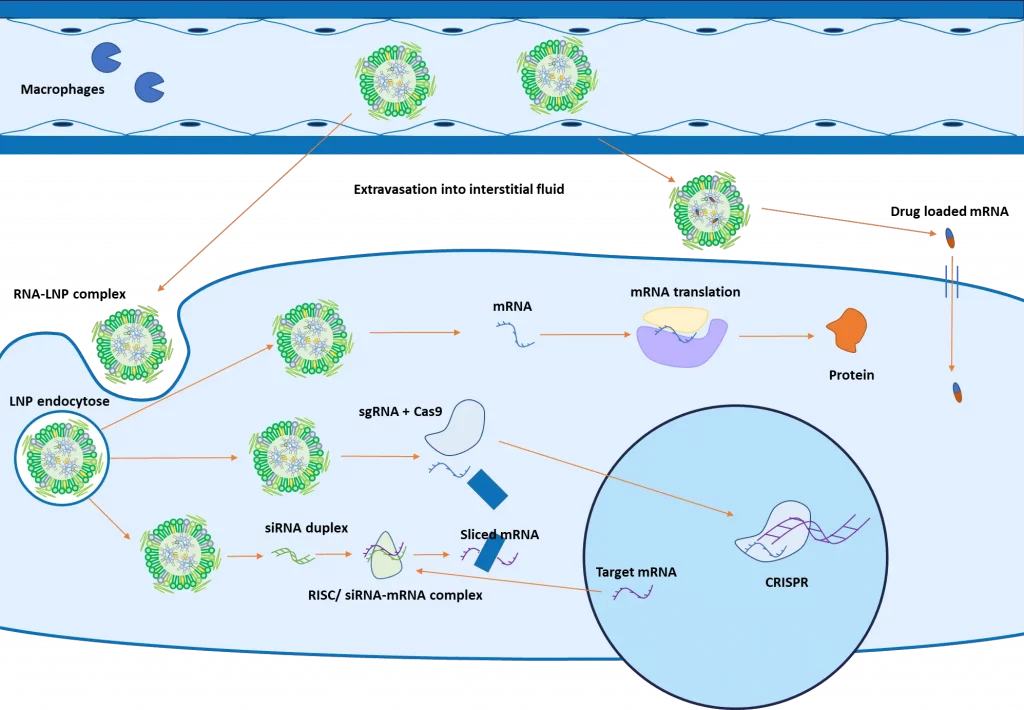
As introduced in the figure above, the next steps will then depend on the type of RNA encapsulated: mRNA will be translated into proteins by the ribosomes in the cytoplasm. SiRNA will combine with other proteins to form a RISC complex, which will bind with a complementary mRNA, inducing its cleavage.
For CRISPR, both the guide RNA and the Cas9 protein are encapsulated and delivered to the cell for gene editing
LNP functionalization
In the previous step, we have described how LNP manages to efficiently release its encapsulated material within the cell. However, an efficient drug not only requires efficient delivery of material but also requires the delivery of the right amount of drug/RNA at the right location.
2 main strategies can be used for nanoparticle targeting: active and passive.
Passive targeting
Passive targeting relies on the careful optimization of the lipid nanoparticle physicochemical parameters (size, EE%, PDI) to improve the accumulation of nanoparticles in some location.
In the example of tumor cells, the careful selection of nanoparticle size allows for an improved cellular uptake by the target cancer cells rathan than regular cells. Additionally, the enhanced vascularization of the tumor leads to a longer retention time within the tumor itself, leading to more extravasation and thus accumulation of the lipid nanoparticle at the location. This process has been widely studied and is known as the enhanced permeability and retention (EPR) effect.However, not all types of diseases show this effect, which can also vary from model to model.
Active targeting
Active targeting relies on the functionnalization of the LNP by a ligand to bind to a specific surface. Those targeting ligands located on the surface of the NP can recognize specific receptors on disease cells, and pathogens… and conjugate with them.
The decoration can be achieved either by the mean of targeting peptides, but also carbohydrate ligands, or even antibodies. This permits a very specific interaction with the tissues, and thus precise delivery of the payload to the cells.
Active targeting assembly methods
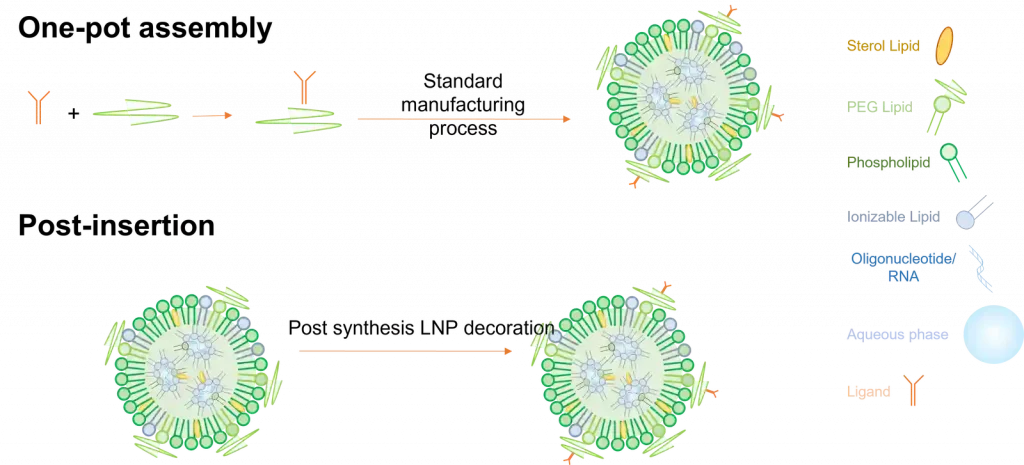
There exist 2 main ways to fabricate functionalized lipid nanoparticles.
The first one called One-pot assembly requires a pre-step where the target ligand is bonded to one of the lipids, which is then used to prepare the LNP using the standard process described in the synthesis method section. Though easy to use, this process shows 2 major drawbacks as most of the ligand can get trapped within the LNP and not at its surface, making it much less efficient. Also, ligands being very fragile, they can very easily be altered during production.
Considering those limitations, ligands are usually post-inserted after their synthesizes.
In this context, they are coated with a lipid with a functional group, which is then put in contact and binds with a targeting ligand. This has the advantage of having the ligands only located on the surface of the LNP but requires a more complex synthesis process.
Lipid nanoparticles applications
Thanks to their unique properties, LNPs have emerged as versatile and promising delivery vehicles in a wide variety of fields, including but not only the pharmaeutical industry. Their improved biodistribution compared to other delivery mechanism and their ability to encapsulate hydrophobic drugs within their lipid bilayers, or RNA/hydrophilic compounds in their aqueous core, makes those delivery system an attractive choice for various applications such as vaccines development or drug delivery.
mRNA Vaccines
mRNA vaccines work by using an mRNA LNP to introduce an mRNA strand that codes for a specific protein, usually a protein of the target virus surface, directly into the cell cytoplasm. While in the cytoplasm, the mRNA will be translated by ribosomes to produce the viral protein it codes for. The immune system will then learn how to recognize this foreign protein and produce the corresponding antibodies. The versatility of this technic makes it a unique tool to treat a wide variety of diseases ranging from infectious ones, such as the covid 19 vaccines, to cancer.
Gene therapy
Gene therapy aims at producing an RNA therapeutics effect by manipulating gene expression to alter the biological properties of a cell. In this specific context, LNP are used to deliver some types of non-coding RNA, such as siRNA, miRNA… that will modify the gene expression within the cell. For instance, siRNA will prevent specific gene expression by degrading its complementary mRNA, preventing translation. Additionnaly, LNP can be used for the delivery of CRISPR-Cas9 compound for gene editing [1]
Drug delivery
In addition to ARN delivery, LNPs have garnered substantial attention in the pharmaceutical industry to encapsulate and deliver drugs, especially for cancer therapies. They are particularly useful for poorly water-soluble drugs, enhancing their solubility, stability, and ultimately, their therapeutic efficacy. LNPs protect drugs from degradation, improve their bioavailability, notably through the EPR effect making it a good fit for tumor targeting, and allow for controlled release, leading to more effective treatments and reduced side effects.
Personalized medicine
The tunable nature of LNPs allows for surface modification with ligands or antibodies that can target specific cells or tissues. This makes it a valuable tool in the development of personalized medicine approaches, tailoring treatments to individual patients' needs.
Agriculture
Lipid nanoparticles can also be used outside medicine. In the context of agriculture, LNPs are used to deliver agrochemicals directly within plants. Their small size and versatile surface modification enable them to penetrate plant tissues more effectively, reducing environmental impact and increasing the efficiency of agrochemical applications.
Interested in learning more about LNP synthesis?
Reach out to our LNP specialist
References
[1] Madigan, V., Zhang, F. & Dahlman, J.E. Drug delivery systems for CRISPR-based genome editors. Nat Rev Drug Discov 22, 875–894 (2023). https://doi.org/10.1038/s41573-023-00762-x
NANOPARTICLE KNOWLEDGE
Looking to learn more about nanoparticle ? Do you need inspiration or a different angle to take on your specific problem? Well, we probably have an application note just for you, feel free to check them out!
Endosomal Escape: A Critical Challenge in LNP-Mediated Therapeutics
Endosomal escape of lipid nanoparticles (LNPs) is a crucial process in the intracellular delivery of mRNA-based therapies and vaccines. After cellular uptake via endocytosis, LNPs must efficiently release their mRNA payload from endosomes into the cytoplasm to ensure proper translation into therapeutic proteins. This step is vital for the success of LNP-mediated delivery systems, as…
Introduction to PLGA Nanoparticles as a Drug Delivery System
For the past decades, modern medicine shifted more and more towards the use of nanocarriers as drug delivery systems (DDS) to overcome the inherent drawbacks of traditional drug administration. To that end, several drug carriers were developed, ranging from lipid nanoparticles (LNPs), and solid nanoparticles (SLNs), to polymersomes, liposomes, and polymeric nanoparticles. On the latter,…
The Evolution of Nanomedicine: from ideas to clinical applications
Nanomedicine represents a transformative approach to diagnostics and therapeutics, leveraging the unique properties of materials at the nanoscale. Its roots extend back to the mid-20th century when the concept of using nanometer-sized particles for medical applications began to materialize. The field has grown significantly over the decades, offering novel solutions in drug delivery, imaging, and…
