Liposomes vs Lipid Nanoparticles as vehicles for delivery applications
As several kinds of lipid-based nanoparticles (LBNPs) are currently available for delivering small molecules, oligonucleotides, and peptide/protein drugs, this review aims at providing a comparison of the 2 most widely used ones: liposomes vs lipid nanoparticles (LNPs).
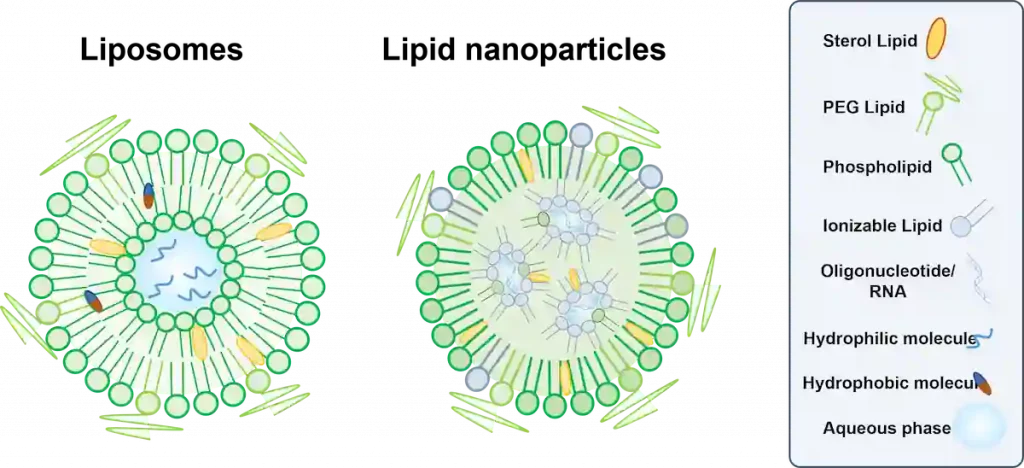
Although mistaken for LNPs, Liposomes are only close cousins of them, rather than ancestors, and present few differences in structure, properties, and hence applications.
This article will provide a detailed description of both elements, with their respective applications. In addition, the main differences between the two nanocarriers, as well as the advantages and drawbacks of each will be highlighted.
What are Liposomes?
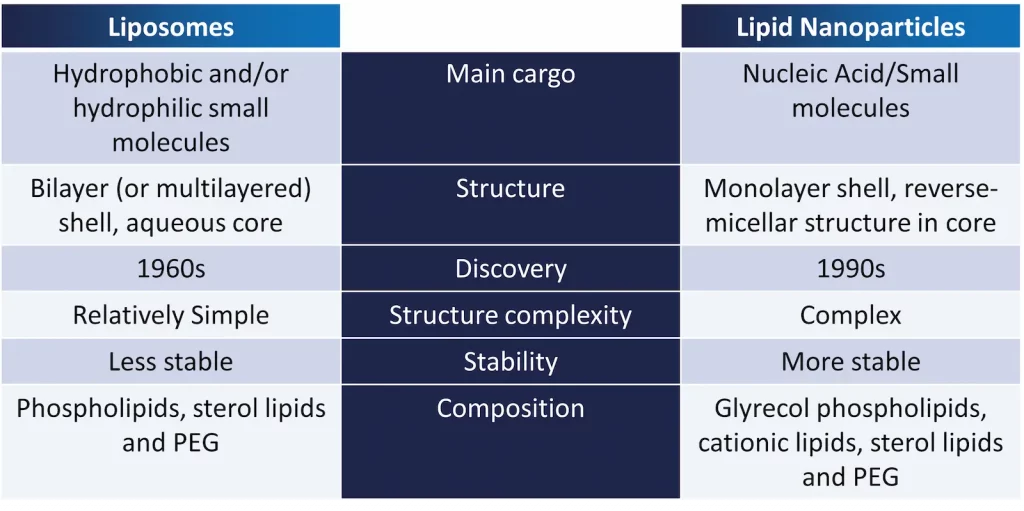
Sometimes referred to as vesicles, they are aggregates composed of a unilamellar (single bilayer) or multilamellar (concentrical bilayers) shell made from amphipathic (amphiphilic) molecules which encloses an aqueous core. Those amphipathic molecules were serendipitously discovered in 1964, and consist of hydrophilic, polar head groups and hydrophobic, non-polar tails, and comprise primarily natural phospholipids (e.g., phosphatidylcholines (PCs), phosphatidylethanolamines (PEs), phosphatidylglycerols (PGs), and phosphatidylserines (PSs)) [B1, B2, B3, B5].
They possess diameters that can range from tens of nanometers up to hundreds of micrometers [B3]. Liposome bilayers may also contain other constituents, such as cholesterol, that act as stabilizers, improving membrane fluidity and reducing membrane permeability [B1, B3]. Accordingly, cargos of appropriate sizes can be enclosed within their inner aqueous compartments (if hydrophilic) or even within their lipid bilayer (if lipophilic). This unique structure allows them to deliver multiple cargos simultaneously.
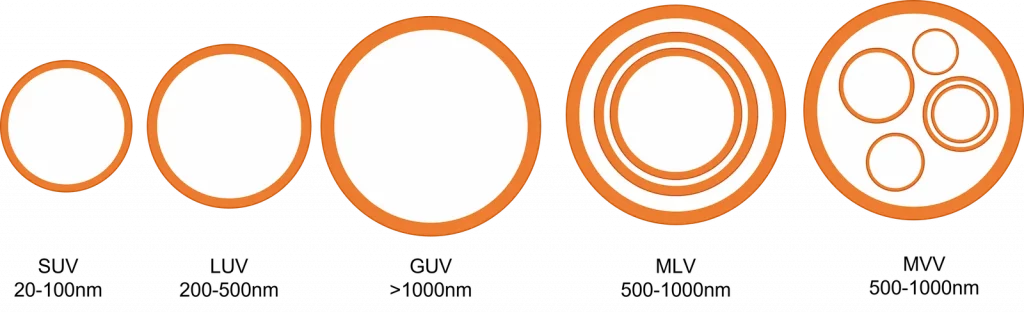
These nanostructures can be categorized depending on their applications or structure, the latter being more commonly used [B5, B6, B7]. Accordingly, they can be subdivided into different types depending on their size and lamellarity [Figure 1], and are generally clustered as such:
- Unilamellar vesicles (ULV): One single lipid bilayer forming one single vesicle.
- Small unilamellar vesicles (SUV).
- Medium unilamellar vesicles (MUV).
- Large unilamellar vesicles (LUV).
- Giant unilamellar vesicles (GUV), are distinguished from the above as having a diameter in the microns scale.
- Oligolamellar vesicles (OLV): few concentric bilayers.
- Multilamellar vesicles (MLV): many concentric bilayers forming an onion-like multilayer structure.
- Multivesicular vesicles (MVV): multiple small vesicles enclosed within a larger vesicle.
Each of these formulations offers different characteristics in terms of drug loading capacity, release profile, and stability. Note that size range classification can vary from one source to the other but are typically in the same order of magnitude.
Applications of Liposomes
Liposome research and development has led to multiple use cases in the biomedical field, with Doxil – a PEGylated MLV carrying chemotherapy cancer drug – being the first to be FDA-approved in 1995 [B1]. Progressively, the advances in formulation and surface functionalization (i.e., PEGylation) enhanced the efficiencies of drug delivery and broadened the application areas of liposomes, many of which are already approved with others awaiting approval. This mostly includes cancer treatments, but also incorporates anti-inflammatory, antibiotic, antifungal, anesthetic, and analgesic drugs, in addition to gene and immuno-suppressant delivery [B6, B7]. Moreover, they have also been used for other applications, such as the cosmetics, biosensing, food, and farming industries [B3, B6].
What are Lipid Nanoparticles?
The term Lipid Nanoparticles (LNPs) started to be used in 1990, after the first laboratory invention and patent filled in 1991, at the time when the era of nanoscience and nanotechnology began [B7]. This term sometimes mistakenly incorporates other nanocarriers such as Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs), which also fall under the umbrella of LBNPs, but differ from LNPs.
In terms of structure, LNPs’ main distinction from other LBNPs, especially Liposomes, is within the structure, having a core that normally encompasses reverse micellar structures, and walled by a lipid monolayer shell [B1]. The monolayer is generally composed of phospholipids, cholesterol – for increased structural stability and adjusting fluidity, enhancing circulation time, in addition to PEGylation – which enhances stability and provides a “stealth-effect”, providing higher circulation half-life too, and ionizable cationic lipids – which are also present in the micellar structure and permit the efficient encapsulation of nucleic acids, making LNPs widely recognized for non-viral gene delivery [B1]. Furthermore, these LNPs are used to deliver most types of nucleic acids (RNA, DNA, peptides…) [B1]. More on LNP can be read in our Lipid nanoparticle introduction.
Applications of Lipid Nanoparticles
The increased need for effective LBNP systems for gene therapy and nucleic acid delivery pushed the development of LNPs forward, especially with the FDA approval of Onpattro (patisiran) in 2018 [B1]. In fact, the characteristic properties of LNPs allow them to be good candidates for carrying and delivering nucleic acid, for potential DNA & RNA-based treatments. Hence, the applications of LNPs as nanocarriers depend on the type of cargo within.
- siRNA LNPs: small interfering RNA (siRNA) targets typically messenger RNA (mRNA) and inhibit the expression of fragments that can cause diseases by specifically preventing protein translation [B1, B8]. This is referred to as an RNA interference (RNAi) therapy, and it is the case of Onpattro, which targets the mRNA that encodes for transthyretin (TTR) protein which is involved in the development of hereditary transthyretin-mediated amyloidosis (hATTR amyloidosis), leading to a peripheral nerve disease [B1, B7]. Long non-coding RNA (lncRNA) is also being investigated as a potential therapeutic target for siRNA LNPs, widening the use cases of LNPs [B1].
- mRNA LNPs: In contrast to siRNA, mRNA induces protein expression. In diseases where genetic mutations cause issues with protein synthesis or for the production of neutralizing antibodies, mRNA-based therapies can be used to restore normal cellular function [B1]. This was the case of the two COVID-19 vaccines developed by Pfizer-BioNTech and Moderna Therapeutics (Comirnaty BNT162b2 and Spikevax mRNA-1273, respectively) to induce an immune response against the SARS-CoV-2 virus [B1, B4, B7, B8]. Following the advances in the mRNA-based COVID-19 vaccine, multiple clinical trials are presently underway using LNP-formulated mRNA drugs and vaccines. Moreover, mRNA vaccines could be potentially used for cancer immunotherapy or even gene editing via the CRISPR/Cas9 system [B1]. mRNA LNPs are promising despite being relatively new in terms of development and very recent in terms of approval [B7].
Other genetic therapeutics are also being investigated, such as microRNA (miRNA), single-guide RNA (sgRNA)-mediated CRISPR-Cas9 system [B8], and plasmid DNA pDNA [B1].
What are the main similarities and differences ?
Falling under the umbrella of LBNPs, these two elements are lipidic structures that can be used as nanocarriers for different types of cargo and therapeutics. They both consist of lipidic shells that protect their cargo, especially those that cannot be delivered in traditional drug delivery systems (DDS). In addition, these two nanostructures are biocompatible and biodegradable, can be PEGylated to permit longer circulation times, benefit from the enhanced permeation and retention (EPR) effect for passive targeting (depending on their size) mainly for cancer, and can be even functionalized to permit active targeting of therapeutic sites. Learn more about nanoparticle targeting strategies in our review.
Finally, these two nanostructures can be synthesized using the same manufacturing processes, such as introduced in our nanoparticle manufacturing review.
However, Liposomes and LNPs have important differences, which are listed in Table 1 below.
Advantages and drawbacks
This final section presents the main differences between liposomes vs LNPs. It is crucial to note however that advantages and drawbacks can highly depend on the drug encapsulated and the administrative route (oral, transdermal, intravenous, etc.).
Liposomes
Heretofore, the research involving the encapsulation of drugs within liposomes has shown measurable advantages in terms of better protection and sustained release of the drugs, in addition to the possibility of carrying multiple hydrophobic and/or hydrophilic drugs at once [5, 6]. The PEGylation of their structure also improved further their circulation times in-vivo, providing a “stealth effect” [2].
Yet, those vesicles faced multiple challenges limiting their success rates. It has been found that the size of liposomes has a great impact on encapsulation efficiency [7] and ADME (absorption, distribution, metabolism, and excretion) pharmacokinetics, specifically in terms of distribution and clearance [5]. For instance, it was identified that size dictates whether they can enter or exit fenestrated vessels in the liver endothelium or tumor microenvironment [5]. Also, apart from the effect of size on the speed of activation of the Mononuclear Phagocyte System (MPS), large liposomes may not be able to leave capillaries that perfuse vital tissues such as the lung, heart, and kidney or may be retained for longer times once inside tissue with narrow pores [5]. In other instances, liposomes of certain size ranges can escape through discontinuous leaky capillaries in the vicinity of some organs, reducing the specificity and increasing the toxicity of those DDS [5]. Other limitations of liposomes include their low physical and chemical stability, low solubility in aqueous solutions, and short half-life in the body environment. Drug leakage from liposomes has also been reported in multiple instances [6]. Besides, cationic liposomes – which were developed to carry and transport negatively charged nucleic acids – have shown a lot of stability and toxicity problems [2, 6, 7]. These cationic liposomes promised since the early 90s the possibility of incorporating DNA as cargo. However, they were found to be toxic to macrophages, and reduced their secretion of important immune modulators as well [6].
LNPs
The discovery of LNPs is expected to resolve many of the challenges faced with liposomes, especially in terms of gene therapy. As mentioned above, LNPs’ lipid structure generally consists of ionizable lipids, which are positively charged at acidic pH (to condense RNAs), and neutral at physiological pH. This permitted the reduction of toxicity – a characteristic drawback of cationic liposomes [4, 8]. In addition, those ionizable lipids enhance encapsulation stability and efficiency of RNAs [1] and offer overall higher bioavailability and lower cytotoxicity, turning them into the most promising nanocarriers for nucleic acids. Furthermore, LNPs have been optimized to enable better cell uptake and cargo release, permitting enhanced delivery [1, 4].
Nevertheless, the research on LNPs is faced with multiple challenges, mainly related to the choice of cationic lipid and size, as it can influence the performance of in-vivo release. As such, a compromise in size should be found to balance cell targeting and intake efficiency with fabrication efficiency as well as cytotoxicity prevention. Finally, before any clinical trials are conducted, a thorough comprehension of all the significant phases of the LNPs’ ADME pharmacokinetics and course within the body, beginning from the site of administration to the discharge of the cargo, is highly necessary [4].
Conclusion on the liposomes vs lipid nanoparticles differences
At first glance, it appears that they are close cousins, which share a lot of similarities in terms of size, functionalization possibilities, composition, and synthesis method. However, when giving them a closer look, it appears that both are used in totally different contexts: Liposomes thanks to their simplicity have very quickly become a widespread tool for the encapsulation and delivery of hydrophilic and small hydrophobic drugs, while LNPs, which are much more complex, offer a unique tool for the delivery of nucleic acid material directly inside cells with high efficiency.
References
- Vogelaar, A. et al. (2023) “Use of microfluidics to prepare lipid-based nanocarriers,” Pharmaceutics, 15(4), p. 1053.
- Safinya, C.R. and Ewert, K.K. (2012) “Liposomes derived from molecular vases,” Nature, 489(7416), pp. 372–374.
- Estanqueiro, M. et al. (2016) “The role of liposomes and lipid nanoparticles in the skin hydration,” Nanobiomaterials in Galenic Formulations and Cosmetics, pp. 297–326.
- “Let’s talk about lipid nanoparticles” (2021) Nature Reviews Materials, 6(2), pp. 99–99.
- Kraft, J.C. et al. (2014) “Emerging research and clinical development trends of liposome and lipid Nanoparticle Drug Delivery Systems,” Journal of Pharmaceutical Sciences, 103(1), pp. 29–52.
- Nakhaei, P. et al. (2021) “Liposomes: Structure, biomedical applications, and stability parameters with emphasis on cholesterol,” Frontiers in Bioengineering and Biotechnology, 9.
- Tenchov, R. et al. (2021) “Lipid nanoparticles─from liposomes to mrna vaccine delivery, a landscape of research diversity and Advancement,” ACS Nano, 15(11), pp. 16982–17015.
- Han, X. et al. (2021) “An ionizable lipid toolbox for RNA delivery,” Nature Communications, 12(1).
