Advancing Lipid Nanoparticle Stability: The Crucial Role of Nanoparticle Purification
Introduction
Amongst the numerous nanoparticle manufacturing methods available, microfluidics synthesis has appeared as the gold standard thanks to its unique ability to synthesize uniform nanoparticle populations, both at very small and very large scales. This solvent-based synthesis process goes beyond the mere microfluidic mixing of solutions but also involves several post-processing steps which are crucial to ensure nanoparticle stability and minimize their toxicity. Through this review, we will focus on the most important processing steps following the nanoparticle generation: the purification of the nanoparticle solution.
To this end, you will learn about the importance of purification during polymeric and lipid nanoparticle fabrication. Subsequently, we will provide a comprehensive overview of the prevailing nanoparticle purification methods including centrifugation/ultrafiltration, Tangential Flow Focusing (TFF), and Dialysis.
The importance of downstream nanoparticle purification
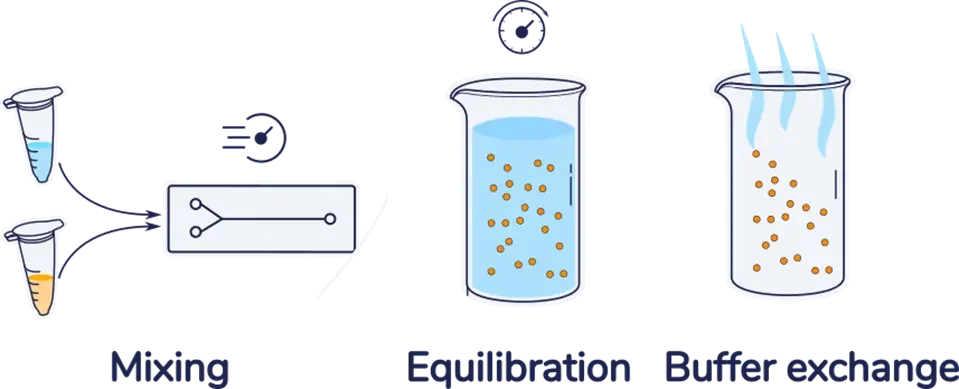
As introduced in our nanoparticle synthesis by microfluidics review, whether it is for Lipid nanoparticles (LNP), liposomes, or lipid-based nanoparticles, the first step of the nanoparticle synthesis process by microfluidics involves the fast and accurate mixing of a solvent solution (Usually ethanol for RNA-LNP) with an anti-solvent solution (typically low pH water) using a micromixer, leading to the nanoparticle formation.
Due to the use of solvent in this core mixing step, the resulting nanoparticles are obtained in a suspension that typically contains 20 to 33% ethanol at low pH, which causes three main limitations:
Stability
The remaining solvent in the solution prevents them from reaching their final organization, making them prone to degradation.
Toxicity
The excess solvent as well as the low pH in the solution would be toxic if used as is on cells.
Purity
There can be some remaining free drug or NP precursor left in the solution, which one wants to remove.
The purification step is therefore crucial to lower the sample toxicity, remove any free drug and NP precursors left, and increase the stability of the nanosuspension.
Nanoparticle purification methods
Numerous methods are available for nanoparticle suspension purification, here is an introduction to the main ones. Before going any further, beware that though necessary this purification step leads to major changes in the critical parameters of the synthesized nanoparticle (size, PDI, EE%), such as illustrated in our article on the importance of dialysis for nanoparticles synthesized by microfluidics.
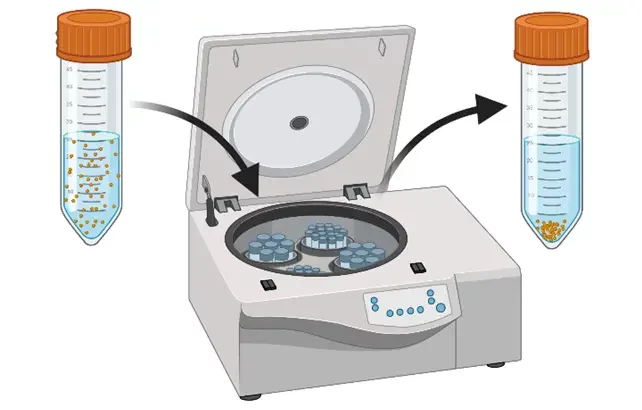
Centrifugation and ultrafiltration
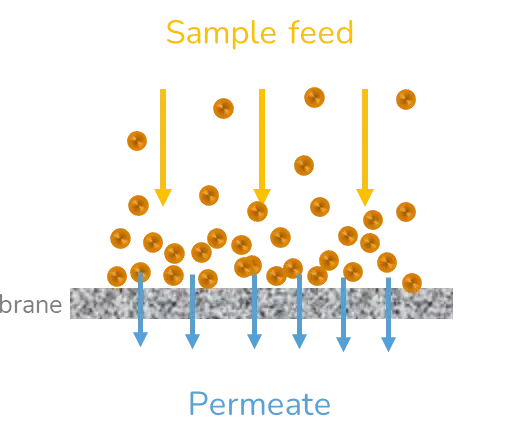
Centrifugation and ultrafiltration use centripetal forces to concentrate NPs into a pellet and separate them from the solvent. In the case of centrifugation, the supernatant is removed by pipetting and the pellet is resuspended in a suitable buffer. Often, this needs to be repeated two or three times to ensure complete removal of the solvent. Ultrafiltration is a membrane-based separation method. The sample is placed on a nanoporous membrane and the tube is subsequently centrifuged to let the solvent flow through the membrane due to the trans-membranous pressure difference. The pellet formed is then resuspended in a clean buffer.
These methods are easy to use but suffer from many drawbacks such as the loss of particles due to surface interactions with the walls of the tubes and/or the membrane and agglomeration due to a concentration polarisation effect. Moreover, they are mostly adapted to relatively small volumes of samples (< 50 mL) and are rarely used in pharmaceutical production settings.
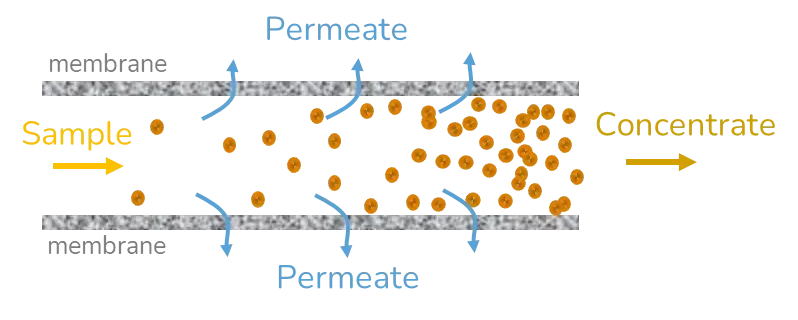
Tangential flow filtration (TFF)
Tangential flow filtration (TFF) is based on the same principles as ultrafiltration, except that this time the sample is flowing through a nanoporous tube and the difference in pressure does not come from the centripetal forces but from the pressure applied to the fluid to drive the flow. The solvent removal efficiency can be enhanced by a retroactive loop reinjecting part of the sample into the inlet feed or by performing multiple cycles of TFF. To counteract the concentration polarization of the sample, a dilution step generally takes place before TFF.
This method is widely used in large-scale production settings as it is very well adapted to process large volumes of samples in a reasonable amount of time. The flow speed, prior dilution of the sample, and number of cycles of TFF need to be optimized to ensure maximal purification of the sample with minimal changes to its physicochemical characteristics and morphology. [2]
Dialysis for Nanoparticle Purification
Dialysis is a purification process that was first developed for blood filtration to replace the natural renal function in patients suffering from renal failure. It can be used for NP purification and assessment of the encapsulation efficiency. The sample is placed in a dialysis bag or cassette made of a semi-permeable nanoporous membrane, typically in regenerated cellulose or polycarbonate (PC), with a size cutoff higher than the size of the NPs. This bag is then placed in a larger volume of buffer until the concentration of solvent and molecules, able to pass through the membrane, reaches an equilibrium state by osmosis. Since the driving force for this process is only diffusion, it is a time-consuming method for sample purification which requires a large amount of buffer. However, it has the advantage of keeping the NP concentration in the sample constant, thus avoiding any agglomeration due to concentration polarization or high shear forces.
To conclude
In conclusion, microfluidics mixing stands out as the most advanced method for synthesizing uniform nanoparticles at varying scales. This process, though effective, poses some limitations due to residual solvents, low pH, and impurities in the synthesized nanoparticles. These challenges can compromise stability, induce toxicity, and diminish purity. To overcome these hurdles, purification emerges as a pivotal step in this regard.
Various methods, such as centrifugation, ultrafiltration, Tangential Flow Focusing (TFF), and dialysis, offer approaches to nanoparticle purification. Each method has its strengths and limitations, catering to different scales of production and purity requirements. It is also important to consider that the purification step also leads to modification in the nanoparticle physicochemical parameters, such as nanoparticle size, PDI...
In essence, the journey from nanoparticle synthesis to application hinges on successful purification. Taking it into account from the start of your RNA vaccines or therapy development process is essential to help mitigate instability and toxicity while enhancing purity.
Ready to Innovate? Reach Out to Us to Your Get your Ultimate Nanoparticle Synthesis Solution!
References
[1] Robertson, James D., Loris Rizzello, Milagros Avila-Olias, Jens Gaitzsch, Claudia Contini, Monika S. Magoń, Stephen A. Renshaw, and Giuseppe Battaglia. "Purification of nanoparticles by size and shape." Scientific reports 6, no. 1 (2016): 1-9. https://www.nature.com/articles/srep27494
[2] Heng-Wen Liu, Yizong Hu, Yong Ren, Hwanhee Nam, Jose Luis Santos, Shirley Ng, Like Gong, Mary Brummet, Christine A Carrington, Christopher G Ullman, Martin G Pomper, Il Minn, Hai-Quan Mao
"Scalable Purification of Plasmid DNA Nanoparticles by Tangential Flow Filtration for Systemic Delivery" Pubmed, 2021 Jul 7;13(26):30326-30336
